Application of Lixisenatide
General description
Lixisenatide (Adlyxin, Sanofi-Aventis) is the newest FDA-approved GLP-1RA and obtained approval on July 27, 2016.
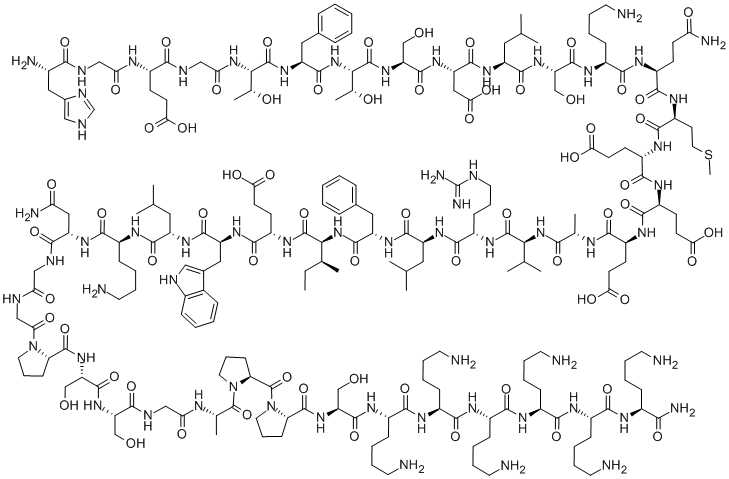
Lixisenatide is a recombinant DNA produced polypeptide analogue of human glucagon-like peptide-1 (GLP-1) which is used in combination with diet and exercise in the therapy of type 2 diabetes, either alone or in combination with other antidiabetic agents. Lixisenatide is a forty-four membered polypeptide consisting of L-His, Gly, L-Glu, Gly, L-Thr, L-Phe, L-Thr, L-Ser, L-Asp, L-Leu, L-Ser, L-Lys, L-Gln, L-Met, L-Glu, L-Glu, L-Glu, L-Ala, L-Val, L-Arg, L-Leu, L-Phe, L-Ile, L-Glu, L-Trp, L-Leu, L-Lys, L-Asn, Gly, Gly, LPro, L-Ser, L-Ser, Gly, L-Ala, L-Pro, L-Pro, L-Ser, L-Lys, L-Lys, L-Lys, L-Lys, L-Lys, and L-Lys-NH2 residues joined in sequence. Used as an adjunct to diet and exercise for the treatment of adults with type II diabetes. It has a role as a glucagon-like peptide-1 receptor agonist, a hypoglycemic agent and a neuroprotective agent. It is a polypeptide and a peptidyl amide.
Application and Pharmacology
Cardiovascular morbidity and mortality are higher among patients with type 2 diabetes, particularly those with concomitant cardiovascular diseases, than in most other populations. We assessed the effects of lixisenatide, a glucagon-like peptide 1–receptor agonist, on cardiovascular outcomes in patients with type 2 diabetes who had had a recent acute coronary event.[1] Lixisenatide is a synthetic GLP-1RA with a binding affinity approximately 4 times greater than that of human GLP-1. In the pancreas, it works by increasing insulin secretion from β-cells in a glucose-dependent manner. Lixisenatide also decreases glucagon secretion from pancreatic α-cells. In the gut, lixisenatide decreases and delays gastric emptying, resulting in increased satiety.The average time to maximum concentrations of lixisenatide following subcutaneous administration is 1 to 3.5 hours. Differences in approved administration sites of the abdomen, thigh, or upper arm did not result in any clinically significant changes in absorption.[2]
Synthesis
A method for synthesizing lisilar. The invention first synthesizes the 1 ‑ 2 amino acid fragment, 3 ‑ 4 amino acid fragment, 29 ‑ 30 amino acid fragment, 32 ‑ 33 amino acid fragment and 34 ‑ 35 amino acid fragment of lisilar respectively, and then synthesizes it according to the peptide sequence from C end to N end of lisilar main chain by fragment and synthesis one by one, The method of the invention carries out the preparation of lisilar by specific fragments and synthesis one by one. On the premise of ensuring high overall yield and purity, the impurities such as [di ‑ his1] ‑ lisilar, [di ‑ Gly] ‑ lisilar, [di ‑ ser33] ‑ lisilar and [di ‑ ala35] ‑ lisilar are reduced by special synthesis strategy, and the product quality of lisilar is improved.[3]
Toxicity and adverse effect
Therapy with lixisenatide has not been associated with serum enzyme elevations or with episodes of clinically apparent liver injury.The 6068 patients who underwent randomization were followed for a median of 25 months. A primary end-point event occurred in 406 patients (13.4%) in the lixisenatide group and in 399 (13.2%) in the placebo group (hazard ratio, 1.02; 95% confidence interval [CI], 0.89 to 1.17), which showed the noninferiority of lixisenatide to placebo (P<0.001) but did not show superiority (P = 0.81). There were no significant between-group differences in the rate of hospitalization for heart failure (hazard ratio in the lixisenatide group, 0.96; 95% CI, 0.75 to 1.23) or the rate of death (hazard ratio, 0.94; 95% CI, 0.78 to 1.13). Lixisenatide was not associated with a higher rate of serious adverse events or severe hypoglycemia, pancreatitis, pancreatic neoplasms, or allergic reactions than was placebo. In patients with type 2 diabetes and a recent acute coronary syndrome, the addition of lixisenatide to usual care did not significantly alter the rate of major cardiovascular events or other serious adverse events.
Reference
1.Pfeffer M. A., Claggett B. & Diaz R. et al., "Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome," The New England journal of medicine, Vol.373, No.23(2015), pp.2247-2257.
2.McCarty D., Coleman M. & Boland C. L., "Lixisenatide: A New Daily GLP-1 Agonist for Type 2 Diabetes Management," Annals of Pharmacotherapy, Vol.51, No.5(2017), pp.401-409.
3.A method for synthesizing Lixisenatide
You may like
Related articles And Qustion
Lastest Price from Lixisenatide manufacturers

US $20.00/box2025-07-24
- CAS:
- 320367-13-3
- Min. Order:
- 1box
- Purity:
- 99%
- Supply Ability:
- in stock

US $20.00/box2025-07-24
- CAS:
- Min. Order:
- 1box
- Purity:
- 99%
- Supply Ability:
- in stock