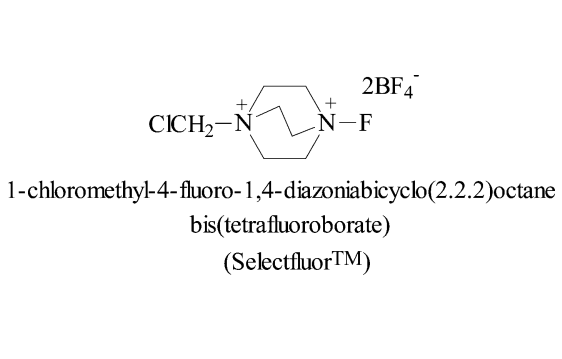
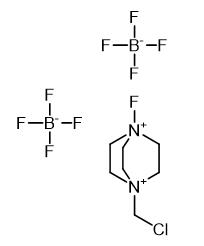
Application and Prevention of 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor(TM) F-TEDA-BF4) is a selective fluoride reagent, which is white crystal at room temperature and has the molecular formula C7H14B2ClF9N2. It is mainly used as a fluorination agent, and can perform monofluorination reaction on electron-rich dibond enol silane enol lithium salt and so on, and is used to prepare fluorosteroidal drugs. Natural selective fluorinated agents are in many ways ideal probes for physiological systems. Because of the presence of trifluoromethyl part in the molecule, the lipophilicity of the molecule is increased, and the transport effect of the fluorinated molecule in the physiological system is improved. The effects of fluoride are thought to inhibit, alternate and enhance normal physiological processes. The vast majority of fluorinated agents generally have high toxicity and high activity, and a lot of work in the field of organic fluorine chemistry often needs to be carried out in laboratories with special conditions.
Physicochemical property
Organic fluoride has been widely used in industry and public life because of its non-toxicity, non-corrosion, chemical stability and other advantages. Fluorine is one of the largest element electronegativity, fluorocarbon bond than carbon atoms combine with other elements to form a single bond is stronger, so bond length is shorter, C - F bonds are hard to break. At the same time, due to the minimum radius of fluorine atoms, that fit the carbon chain skeleton clinging, played a good shielding protection, making them susceptible to the attack of the other atoms in chemical reactions. The stability of organic fluorides increases with the number of fluorine atoms attached to the same carbon atom. It is found that fluoromethane is a kind of useful selective fluorination reagent.
Application
As an efficient and universal electrophilic fluorine source
Selective direct introduction of an iodine atom into alkyl-substituted benzene derivatives was effectively achieved by reaction of target molecules with elemental iodine in the presence of 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo [2.2.2] octane bis(tetrafluoroborate) (Selectfluor(TM) F-TEDA-BF4). The number of iodine atoms introduced could be modulated by the molar ratio between substrate, iodine and F-TEDA-BF4 [1].
Selective and efficient synthesis of alpha,alpha-difluoro ketones was achieved following a protocol which includes the transformation of alpha-methylene ketones to the corresponding n-butylimine derivatives and their further treatment with Selectfluor (TM) F-TEDA-BF4 in acetonitrile solution at 80 degrees C [2].
An effective synthetic pathway for direct introduction of a perfluoroalkyl moiety-containing functional groups at the benzylic position in hexamethylbenzene involved treatment of 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor(TM) F-TEDA-BF4) with hexamethylbenzene in the presence of polyfluoro alcohols or potassium salts of perfluoroalkane carboxylic acids [3].
Reactions of aryl alkyl ketones with methanol solution of elemental iodine and 1-fluoro-4-chloromethyl-1,4-diazoniabicyclo[2.2.2] octane bis(tetrafluoroborate) (Selectfluor(TM) F-TEDA-BF4) result in the formation of corresponding alpha-iodo ketones, while switch over of the regioselectivity can be directed by using acetonitrile as the solvent and selective iodination of the aromatic site of target molecules is thus achieved [4].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
[1] Stavber S, Kralj P, Zupan M. Selective and Effective Iodination of Alkyl-substituted Benzenes with Elemental Iodine Activated by SelectfluorΤΜ F-TEDA-BF4[J]. Synlett, 2002, 2002(04): 0598-0600.
[2] Pravst I, Zupan M, Stavber S. Efficient Synthesis of α, α-Difluoro Ketones Using SelectfluorTM F-TEDA-BF4[J]. Synthesis, 2005, 2005(18): 3140-3146.
[3] Stavber S, Kralj P, Zupan M. SelectfluorTM F-TEDA-BF4 Mediated Introduction of Perfluoroalkyl-containing Groups in the Benzylic Position of Hexamethylbenzene[J]. Acta Chim. Slov, 2002, 49: 553-560.
[4] Stavber S, Jereb M, Zupan M. Selectfluor TM F-TEDA-BF 4 mediated and solvent directed iodination of aryl alkyl ketones using elemental iodine[J]. Chemical communications, 2002 (5): 488-489.
See also
Lastest Price from 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) manufacturers
![140681-55-6 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)](/ProductImageEN/2022-10/Small/9d1278e5-60f3-40dc-9858-3efdad0fae6b.png)
US $0.00-0.00/kg2025-04-04
- CAS:
- 140681-55-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
![140681-55-6 -Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)](/ProductImageEN/2023-10/Small/b094eeb6-56b9-4cab-979e-40f8584b9f05.gif)
US $0.00/kg2023-10-21
- CAS:
- 140681-55-6
- Min. Order:
- 25kg
- Purity:
- 99.0%
- Supply Ability:
- 20mt
![140681-55-6 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); Application; Prevention](https://www.chemicalbook.com/CAS/GIF/140681-55-6.gif)