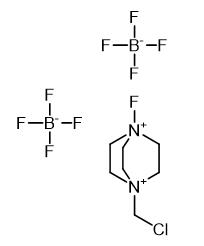
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate): Properties and Synthesis
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) is a stable, non-volatile, and powerful electrophilic fluorination reagent. The electrophilic fluorination reaction is one of the most direct ways to selectively introduce fluorine into organic compounds.
![1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) Article illustration](/NewsImg/2024-06-14/6385398320830245665208832.png)
Selectfluor is a reactive source of an electrophilic [F+] species that interacts with organic, inorganic, and biological molecules. It can be reacted with aromatic, aliphatic, alkene, amine, glycol, and silicon compounds. Most of the reactions have been performed in acetonitrile, but DMF and DMA have also been used. Reaction conditions depend on the substrate used and the nature of the products formed.
Properties of 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) is a white solid (mp 190 °C), stable in air and to moisture. The X-ray crystal structure has been reported. It is a dicationic salt that is very soluble in cold water or dilute hydrochloric acid. However, it decomposes in dilute sodium hydroxide and reacts with cold DMSO (rapidly and exothermally) and with DMF (slowly on heating). It is moderately soluble in acetonitrile, but only slightly soluble in lower alcohols and acetone, which makes the former the solvent of choice. Occasionally, reactions in these solvents were accelerated by adding a small amount of water or trifluoroacetic acid. Bulk quantities of 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) should be stored in a cool, dry place and should not be heated above 80 °C. Fainzil’berg et al. have measured the reduction potentials of Selectfluor and other N-F compounds. With a positive reduction potential, E1/2 ) 0.33 V, Selectfluor is predicted to be a more reactive fluorinating reagent than, e.g., N-fluorobenzenesulfonimide, with E1/2 ) -1.24 V.
Synthesis of 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)
1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) is prepared by the reaction of 1,4-Diazabicyclo[2.2.2]octane (triethylenediamine) and dichloromethane.
![1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) synthesis Article illustration](/NewsImg/2024-06-14/6385398234559104843007145.png)
References:
[1] RAJENDRA P. SINGH; Jean’ne M S. Recent Highlights in Electrophilic Fluorination with 1-Chloromethyl-4-fluoro- 1,4-diazoniabicyclo[2.2.2]octane Bis(tetrafluoroborate)[J]. Accounts of Chemical Research, 2003. DOI:10.1021/ar030043v.
[2] R.ERIC BANKS. N-Halogeno compounds part 17. Precursors of NF-TEDA reagents: quaternary salts of 1,4-diazabicyclooctane containing fluoro-anions, and their Lewis acid-Lewis base adducts with boron trifluoride, phosphorus pentafluoride and sulphur trioxide[J]. Journal of Fluorine Chemistry, 1996. DOI:10.1016/0022-1139(96)03395-7.
You may like
See also
Lastest Price from 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) manufacturers
![140681-55-6 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)](/ProductImageEN/2022-10/Small/9d1278e5-60f3-40dc-9858-3efdad0fae6b.png)
US $0.00-0.00/kg2025-04-04
- CAS:
- 140681-55-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
![140681-55-6 -Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)](/ProductImageEN/2023-10/Small/b094eeb6-56b9-4cab-979e-40f8584b9f05.gif)
US $0.00/kg2023-10-21
- CAS:
- 140681-55-6
- Min. Order:
- 25kg
- Purity:
- 99.0%
- Supply Ability:
- 20mt
![140681-55-6 1-ChloroMethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)PropertiesSynthesis](https://www.chemicalbook.com/CAS/GIF/140681-55-6.gif)