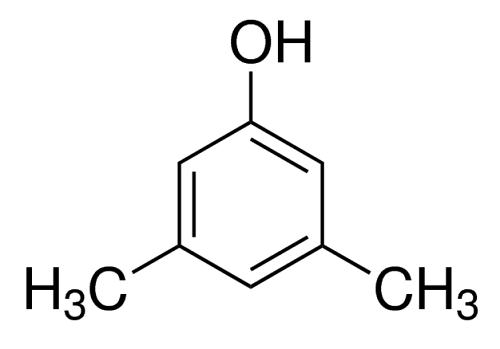
Application and preparation of 3,5-dimethylphenol
3,5-dimethylphenol is a colorless or yellowish needle like crystal, slightly soluble in water and soluble in organic solvents such as sodium hydroxide solution and ethanol. It is toxic and corrosive, and can cause poisoning by inhalation through mouth and skin. The LD50 of Groundhog was 1750mg / kg. Main physical parameters: melting point 68 ℃, boiling point 219.5 ℃.
Application
3,5-dimethylphenol is an important industrial intermediate, which is mainly used to prepare antioxidants, antibiotics, resin adhesives and vitamin E. Nowadays, the demand for 3,5-dimethylphenol is increasing year by year in the world, and the existing production process not only pollutes the environment seriously but also has low purity. Therefore, it is of great significance to develop a production route of 3,5-dimethylphenol with little pollution.
3,5-xylidine is an important raw material for pigment synthesis. The traditional preparation process of 3,5-dimethylaniline takes 2,4-dimethylaniline as raw material and is prepared through acylation, nitrification, hydrolysis, diazotization, reduction and other processes. This process is characterized by long process, low yield, high cost and certain difficulties in industrial production. The literature reported the principle of preparing 2,6-dimethylaniline by ammoniating 2,6-dimethylphenol. 3,5-dimethylaniline was prepared from 3,5-dimethylphenol by partial hydrogenation and re ammoniation, which shortened the process flow and provided a basis for further industrial production[1].
Isophorone reacts at high temperature. Besides 3,5-dimethylphenol and CH4, isophorone is also involved in the decomposition of Isophorone to obtain a variety of products. Isopropylidene acetone and acetone isopropylidene acetone are the first products of Isophorone decomposition. Isopropylidene acetone can hydrate with water adsorbed on the surface of the catalyst to form diacetone alcohol. Diacetone can be decomposed to isobutylene and acetic acid or acetone on the catalyst. Condensation dehydration of isopropylidene acetone with acetone can produce 1,3,5-trimethylbenzene and isophorone. The condensation of isopropylidene acetone itself can produce a variety of isoxylones, while isopropylidene acetone and isophorone can produce tetrahydronaphthalene ketone. It shows that 3,5-dimethylphenol and many by-products can be produced simultaneously in the reaction of Isophorone at high temperature. It is important to improve the selectivity of 3,5-dimethylphenol by selecting suitable catalysts[2].
The preparation of 3,5-dimethylphenol
For the preparation of 3,5-dimethylphenol by catalytic demethylation of isophorone in the gas phase in the presence of a metal or a metal alloy as catalyst, an isophorone-containing process stream is guided through reaction zones that contain said catalyst, whereby the reactivity of the catalyst varies in the reaction zones and the process stream between these reaction zones is thermostated in zones that do nor contain any catalyst, whereby the residual isophorone is greatly reduced, and the yield of 3,5-dimethylphenol is increased[3].
Processing technology for extracting 3,4-dimethylphenol and 3,5-dimethylphenol
The invention discloses a processing technology for extracting 3,4-dimethylphenol and 3,5-dimethylphenol. The processing technology for extracting 3,4-dimethylphenol and 3,5-dimethylphenol includes the following steps: collecting mixed industry xylenols, specifically, centralizing and collecting mixed industrial xylenols by workers, then putting the collected mixed industrial xylenols in a storagetank for subsequent use; conducting rectification for purifying 3,5-dimethylphenol; conducting an alkylation reaction; conducting a crystallization separation reaction; storing 3,5-dimethylphenol and3,4-dimethylphenol separately; and conducting quality inspection. The processing technology for extracting 3,4-dimethylphenol and 3,5-dimethylphenol from the mixed industrial xylenols is simple to operate and convenient for purifying 3,4-dimethylphenol with a content of 8-10% and 3,5 dimethylphenol with a content of 35-45% in the mixed xylenols to 99.5% or more, greatly improves the quality of the products as a whole, has the performance of high purity, high yield and suitability for large-scale production, has good market value, has a broad market prospect, and is suitable for promotion[4].
Reference
1 Wang Wanqin, Zhang Xiucheng, Chen Liyu, Dong Wu. Study on the process of synthesizing 3,5-dimethylaniline by ammoniation [J]. Dye Industry, 2001 (03): 35-36
2 Zhang Xian, Wang Rijie, Yu Jingang, Kong Haining, Tian Guilin. Research progress in the aromatization of isophorone to 3,5-dimethylphenol [J]. Chemical Industry and Engineering, 2005 (06): 472-475
3 US5969196A Process for preparing 3,5-dimethylphenol
4 CN110551006A Processing technology for extracting 3,4-dimethylphenol and 3,5-dimethylphenol
You may like
See also
Lastest Price from 3,5-Dimethylphenol manufacturers

US $1.00/KG2025-04-21
- CAS:
- 108-68-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-15
- CAS:
- 108-68-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
![166388-57-4 2-[2-(2-Azidoethoxy)ethoxy]ethanaminepreparationsynthesis intermediate](/NewsImg/2022-10-12/6380118556579815325808950.jpg)
