Anthracene: A Versatile Polycyclic Aromatic Hydrocarbon
Introduction
Anthracene, a solid polycyclic aromatic hydrocarbon (PAH), consists of three fused benzene rings in a linear arrangement. Known for its brilliant fluorescence and substantial role in organic chemistry, anthracene serves as a cornerstone in the study and application of aromatic compounds. Its unique structure makes it ideal for various chemical manipulations, leading to numerous industrial and scientific applications. This article will explore the synthesis, composition, applications, and storage methods of anthracene, underscoring its critical importance and versatile nature in the chemical industry. From its origins in coal tar to its utilization in high-tech applications, anthracene's journey reflects significant advancements in chemical processing and material science, making it a subject of interest for professionals in chemistry and related fields[1].

Figure 1 Characteristics of Anthracene
Synthesis of Anthracene
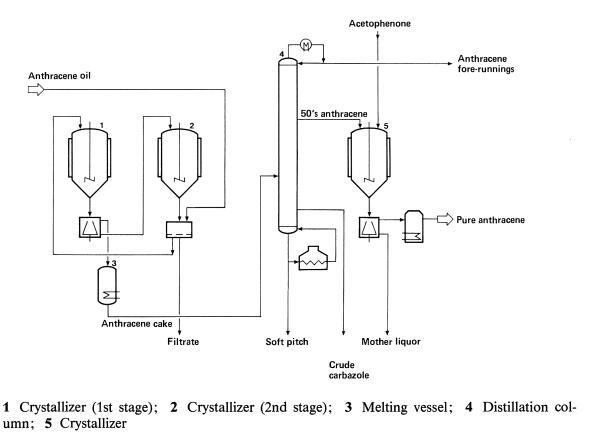
Anthracene is predominantly derived from coal tar, a byproduct of coal processing. The extraction process involves the distillation of coal tar at temperatures between 300°C and 400°C, during which anthracene oil is separated. The oil contains about 1.5% anthracene along with other aromatic hydrocarbons. Further purification is achieved through recrystallization from solvents such as benzene or toluene, yielding anthracene of high purity suitable for industrial use.
Recent synthetic methods have also been developed to produce anthracene more sustainably. These include the dehydrogenation of alkylbenzenes and the cyclization of stilbenes under controlled conditions. Such synthetic routes are particularly valuable for producing anthracene with specific isotopic or structural modifications for research applications.
Main Constituents
Anthracene itself is a relatively simple molecule, but its derivatives are structurally diverse and functionally rich. Derivatives such as anthraquinone are critical in dye manufacture, while others are used in the synthesis of drugs and advanced organic materials. The molecular structure of anthracene allows for various substitutions, which can modify its chemical and physical properties dramatically.
Applications of Anthracene
The applications of anthracene are diverse, ranging from the production of dyes and pigments to its use in organic light-emitting diodes (OLEDs). In the pharmaceutical industry, derivatives of anthracene are employed in the synthesis of several drugs, highlighting its importance in medicinal chemistry.
Anthracene's photoreactive properties make it a staple in research laboratories studying the mechanisms of photochemistry. Its ability to form excited states and react with other molecules under light exposure is utilized in studies of molecular behavior, which is crucial for developing new photoreactive materials.
Furthermore, anthracene is used in the calibration of ultraviolet (UV) light instruments due to its consistent fluorescence characteristics. This application is vital in ensuring the accuracy and reliability of UV-based analytical devices.
Storage Methods
Proper storage of anthracene is crucial to maintain its chemical integrity and ensure safety. Anthracene should be stored in a cool, dry place away from light to prevent degradation and preserve its reactivity. It must be handled under inert atmospheres, typically nitrogen, to prevent oxidation. Containers used for storing anthracene should be tightly sealed to avoid contamination with moisture or other substances that could affect its purity and efficacy. Moreover, the storage area should be regularly inspected for any signs of leaks or damage to containers.
Given its classification as a potential carcinogen, safety measures during handling and storage are stringent. Facilities storing large quantities of anthracene are equipped with appropriate ventilation systems to minimize exposure to its dust, which can be hazardous if inhaled. To further ensure safety, personnel must employ protective gear, including masks and gloves, and follow strict protocols when interacting with this chemical.
Conclusion
Anthracene is a compound of immense scientific and industrial value, playing a pivotal role in various sectors, including pharmaceuticals, material science, and chemical analysis. Its ability to be synthesized from coal tar and modified through chemical reactions makes it a versatile and indispensable resource in the chemical industry. As research continues to uncover new properties and applications of this fascinating molecule, anthracene's relevance is sure to grow, highlighting its enduring significance in the advancement of science and technology[2].
References
[1]Yoshizawa M, Klosterman J K. Molecular architectures of multi-anthracene assemblies[J]. Chemical Society Reviews, 2014, 43(6): 1885-1898.
[2]Van Damme J, Du Prez F. Anthracene-containing polymers toward high-end applications[J]. Progress in polymer science, 2018, 82: 92-119.
You may like
Related articles And Qustion
Lastest Price from Anthracene manufacturers

US $9.00/KG2024-05-29
- CAS:
- 120-12-7
- Min. Order:
- 1KG
- Purity:
- 99.50%
- Supply Ability:
- 50000tons
US $50.00-1.00/KG2024-03-25
- CAS:
- 120-12-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available