Triacetin: Versatile Acetylated Glyceryl Ester in Modern Chemistry
Introduction
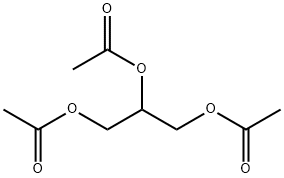
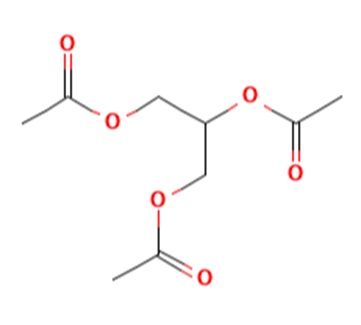
Triacetin, also known scientifically as glyceryl triacetate, is a clear, colorless, and odorless liquid that plays a pivotal role in various chemical and industrial applications. Its chemical formula, C_9H_14O_6, reflects a structure where three acetate groups are bonded to a glycerol backbone, making it a triglyceride that is both simple and versatile. This article provides an in-depth look into the synthesis, composition, applications, and storage of triacetin, aimed at professionals in the chemical industry seeking comprehensive knowledge about this important compound.

Figure 1 Characteristics of Triacetin
Synthesis of Triacetin
The synthesis of triacetin primarily involves the esterification of glycerol with acetic acid. This reaction is typically catalyzed by acidic or basic catalysts and can be conducted under reflux to ensure complete conversion. The process may vary slightly depending on the desired purity and yield, with industrial synthesis often scaling up this reaction under controlled conditions. Advances in catalytic systems and process engineering have enabled more efficient and environmentally friendly production methods, which are crucial in meeting the growing demand for triacetin in various sectors.
Main Components and Chemical Properties
Triacetin is a triester composed of glycerol and acetic acid. The ester bonds in triacetin give it unique properties such as a high boiling point, low volatility, and excellent solubility in common organic solvents. These properties make triacetin not only effective as a plasticizer but also valuable in applications requiring a stable, non-volatile solvent. Furthermore, its biodegradability and low toxicity profile enhance its appeal in applications that prioritize environmental safety and human health.
Uses of Triacetin
Triacetin's applications are diverse, reflecting its utility in various industrial and chemical processes. It is extensively used as a plasticizer in cellulose-based plastics, providing flexibility and durability to products such as film coatings and tool handles. In the pharmaceutical industry, triacetin serves as a solvent and excipient, aiding in the formulation of capsules and tablets by enhancing the dissolution of active ingredients and improving their bioavailability. Its fungicidal properties are leveraged in agricultural applications, particularly in fungicides and pesticides, where it acts as an effective carrier that helps in the controlled release of active agents. Additionally, triacetin is employed as a food additive, acting as a flavor carrier and food contact material, used to enhance flavors in baked goods and confectioneries. Its role in the production of smokeless gunpowder highlights its importance in defense applications, where it is valued for its stabilizing and plasticizing properties, contributing to the consistency and performance of propellants.
Conclusion
Triacetin is a byproduct obtained from the reaction between triglycerides and methyl acetate (interesterification) and has a higher added value than that of glycerol. According to the reaction stoichiometry, the final product contains 20 wt % of triacetin and 80 wt % of biodiesel in a single phase. The aim of this work was to test the performance of biodiesel quality standards when triacetin is present at different concentrations in the biodiesel. We therefore measured properties, such as the density, kinematic viscosity, cloud point, pour point, cold filter plugging point, dynamic viscosity, cetane number, heating value, distillation curve, and flash point, for mixtures of triacetin and biodiesel composed of various amounts of triacetin of up to 20 wt % and different biodiesels (palm, soybean, sunflower, high-oleic sunflower, and rapeseed). Current biodiesel fuel standards would limit the triacetin to about 10 wt % (EN 14214) if they considered that the effect of adding triacetin is different to that of conventional triglycerides. There would not be any restriction for amounts of up to 20 wt % according to the American Society for Testing and Materials (ASTM) D6751 guidelines.
![Article illustration]() References
References
[1]Casas A, Ruiz J R, Ramos M J, et al. Effects of triacetin on biodiesel quality[J]. Energy & Fuels, 2010, 24(8): 4481-4489.
[2]Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis[J]. The journal of physical chemistry A, 2012, 116(18): 4602-4609.
Related articles And Qustion
See also
Lastest Price from Triacetin manufacturers

US $100.00-80.00/KG2025-07-15
- CAS:
- 102-76-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000000

US $320.00/kg2025-05-26
- CAS:
- 102-76-1
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 18400kg