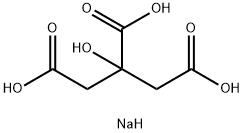
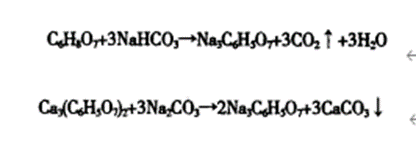Sodium Citrate: An Indispensable Component in Modern Chemistry
Introduction
commercial sodium citrate (SC) is proposed as a low-cost, environmentally friendly, high-efficiency self-sacrificial additive for the first time. Rich sodiated carboxyl groups enable SC to deliver a considerable capacity of 312 mA h g-1 with a capacity utilization of 97% for Na compensation. The electrochemical oxidation decomposition mechanism of SC has been preliminarily discussed. More importantly, the appropriate addition of SC successfully increases the reversible energy density of the as-constructed full cell with negligible negative effects. Commercially available SC was directly used in the experiment without further treatment. In the scanning electron microscopy (SEM) image, SC is composed of heavily aggregated particles with several hundred nanometers in size. Each SC molecule possesses three sodiated carboxyl groups and delivers a considerable theoretical capacity of 312 mA h g-1.

Test principle
Sodium citrate is a salt of citric acid, a simple organic compound found as one of the metabolites in the Krebs cycle. Some bacteria can obtain energy by using citrate as the sole carbon source. This is an important test for identifying many bacterial species and the differentiation of Rapid Growers. Any medium used to detect citrate utilization by test bacteria must be devoid of protein and carbohydrates as carbon sources. The test medium contains sodium citrate as the sole source of carbon and ammonium phosphate as the sole nitrogen source. The bacteria that can utilize citrate can also extract nitrogen from the ammonium salt, which leads to the production of ammonia and the alkalinisation of the medium, thereby turning the bromothymol blue indicator to blue.
Uses
Sodium citrate is commonly used as an emulsifier for oils. It is used in food industries as an acidity regulator and sanitizer by lowering the pH, providing unsuitable conditions for bacterial growth. It is also used in the collection of blood samples to prevent clotting in storage. Furthermore, sodium citrate is used to neutralize excess acid in the urine and blood and treat chronic kidney diseases and metabolic acidosis. Importantly, it was shown that sodium citrate has antimicrobial activity, independent of pH, against oral Streptococcus pneumoniae and several oral bacteria such as Fusobacterium nucleatum and Streptococcus mutans. Importantly, it was reported that sodium citrate (4%) could inhibit the formation of Klebsiella pneumoniae biofilms by 46.5%. In another study, 4% sodium citrate in solution with 0.0015% nitroglycerin and 22% ethanol could eradicate biofilm formed by methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), vancomycin-resistant enterococci (VRE), multidrug-resistant Klebsiella pneumoniae, P. aeruginosa, Acinetobacter baumannii, Enterobacter cloacae, Escherichia coli, and Stenotrophomonas maltophilia, in addition to Candida albicans and Candida glabrata. Furthermore, sodium citrate at a concentration of 4% was able to prevent the biofilm formation of K. pneumoniae, Staphylococcus aureus, and Escherichia coli.
Sodium citrate, commonly used as an environmentally friendly reducing reagent for synthesising nanoparticles (NPs), can convert GO to reduced graphene oxide nanosheets (RGONS). More importantly, Au nanoparticle–reduced graphene oxide nanosheet (Au– RGONS) hybrid materials can be easily obtained using sodium citrate as a reductant and stabilizer in a one-pot reaction. This novel and simple synthesis route to RGONS and Au–RGONS hybrid materials has several important benefits: (1) Sodium citrate is simultaneously used as an environmentally friendly reducing agent and as a capping reagent for the effective reduction of both GO and Au precursors and for stabilization of the corresponding reduced products; (2) sodium citrate-protected Au–RGONS hybrid materials with good size distribution of Au NPs can be synthesized in one-pot; (3) Au NPs can form a uniform distribution on the surface of RGONS without the formation of agglomerates.;
Citrate toxicity
Sodium citrate is the anticoagulant of choice used in blood collection. In a massive transfusion, an excessive amount of citrate can produce transient hypocalcaemia and hypomagnesaemia that may affect the cardiac rate and function. This is usually seen only in patients with liver failure and/or severe hypothermia, where citrate metabolism is slowed. Treatment with calcium and, rarely, magnesium is tailored to clinical findings and ion levels.
References
[1] Zhang, Rui et al. “Sodium citrate as a self-sacrificial sodium compensation additive for sodium-ion batteries†.” Chemical Communications 35 (2021): 4243–4246.
[2] M. Khayat. “Sodium Citrate Alleviates Virulence in Pseudomonas aeruginosa.” Microorganisms (2022).
Related articles And Qustion
Lastest Price from Sodium citrate manufacturers

US $0.00-0.00/KG2025-12-12
- CAS:
- 68-04-2
- Min. Order:
- 1KG
- Purity:
- 99.0-101%
- Supply Ability:
- 500kg/month

US $1200.00-1100.00/ton2025-08-22
- CAS:
- 68-04-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M