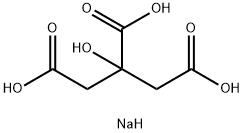
Introduction of Sodium citrate
General description
Sodium citrate is the trisodium salt of citric acid. It has a role as a flavouring agent and an anticoagulant. It contains a citrate(3-). Sodium citrate is the sodium salt of citric acid. It is white, crystalline powder or white, granular crystals, slightly deliquescent in moist air, freely soluble in water, practically insoluble in alcohol. Like citric acid, it has a sour taste. From the medical point of view, it is used as alkalinizing agent. It works by neutralizing excess acid in the blood and urine. It has been indicated for the treatment of metabolic acidosis. Sodium salts of citric acid that are used as buffers and food preservatives. They are used medically as anticoagulants in stored blood, and for urine alkalization in the prevention of KIDNEY STONES.
Application and Pharmacology
Sodium citrate, also known as sodium citrate, is chemically called trisodium 2-hydroxypropyl tricarboxylate. Its molecular formula is c6h5o7 na3 · 2H2O. It is crystal water containing two molecules, white crystal or granular powder. It is stable at room temperature and in air, and loses crystal water when heated to 150 ℃. Since the successful development and industrial production of citric acid fermentation process in the United States in 1923, the earliest deep-processing citrate is sodium citrate. Sodium citrate is an important raw material in industry and is widely used. Sodium citrate is used in food additives, which is in great demand. It is mainly used as condiment, buffer, emulsifier and stabilizer. In medicine, sodium citrate is also widely used. It can be used as blood transfusion agent, soluble calcium ion complex and blood transfusion agent. Sodium citrate injection, like potassium salt, is used to correct the acidity of blood, body fluid and urine as diuretics, expectorants, etc
Blood withdrawn from healthy donors, using blood gas analyses, measurement of ionized calcium (iCa++) concentration, activated clotting time (ACT), thromboelastography (TEG) and multiplate aggregometry, we compared the anticoagulant and acidifying properties of citric acid, lactic acid and sodium citrate. Sodium citrate did not affect blood gas analysis. Contrarily, citric acid and lactic acid at increasing doses determined similar progressive reductions in pH and HCO3- and an increment in pCO2[1].
Synthesis
1.This method is an improved preparation method for soda ash products that are not suitable for the pharmaceutical industry. In this method, high-quality baking soda is dissolved in water according to the calculated amount and neutralized with citric acid to prepare drug grade sodium citrate through concentration and crystallization. It is characterized by mild reaction conditions, good product quality and good process operability. At present, this method is mainly used in some pharmaceutical factories.
2.In this method, sodium citrate is obtained by mixing calcium citrate and soda ash in the neutralization process of citric acid production enterprises, filtering and removing insoluble substances. The purity of the product is poor and the process operation process is long. A few years ago, it was reported that by adjusting the pH value of mixing conditions, the process flow was simplified, the production cost was reduced and the products with better quality were obtained[2,3]. The reaction chemical equation is:
Figure 1 The reaction chemical equation of Sodium citrate
Safety
Sodium citrate has been reported to improve the performance of short duration, high-intensity exercise in some, but not all studies, raisingdoubts regarding its ergogenic efficacy. The reasons for the inconsistent results across previous studies are unclear, but may be attributable to suboptimal ingestion protocols, which may not allow sufficient time for peak blood alkalosis (increased blood pH, typically coinciding with an increased blood bicarbonate concentration ([HCO3-])) to occur, potentially limiting the ergogenic benefit (Potteiger et al. 1996). Further, GI symptoms such as nausea and bloating have been reported after sodium citrate ingestion and may limit the performance of affected participants. Therefore, there is a need to identify an ingestion protocol which induces alkalosis and minimizes GI symptoms with the aim of improving high-intensity exercise performance. The most effective sodium citrate dose (500 mg/kg-1 body mass (BM)) has been established based on induced alkalosis, gastrointestinal, and performance outcomes (McNaughton 1990; Urwin et al. 2016); however, induced alkalosis after sodium citrate ingestion may be further affected by ingestion mode. Recent findings indicated that sodium bicarbonate ingested in polymer-coated, gastro resistant capsules elicited a significant delay in peak alkalosis compared to the same dose ingested in a solution as well as a lower incidence and severity of GI disturbances.
Improved exercise performance following sodium citrate supplementation may alternatively be attributed to elevated plasma volume and subsequent improvements in muscle perfusion associated with an increased blood sodium concentration. To date, the ergogenic capacity of sodium citrate has not been studied to the same extent as that of sodium bicarbonate, so the comparative efficacy of the optimized ingestion protocols for the respective supplements is not yet known. The two supplements induce similar levels of blood alkalosis; however, sodium citrate may be a preferable supplement, because it appears to be linked to fewer postingestion gastrointestinal symptoms. Further research is required to confirm this link[4,5].
References
1.Getz T. M., Turgeon A. & Wagner S. J., "Sodium citrate contributes to the platelet storage lesion," Transfusion, Vol.59, No.6(2019), pp.2103-2112.
2.Zhou Benhua: Study on application and preparation method of sodium citrate, Journal of Yancheng Institute of Technology (NATURAL SCIENCE EDITION), 2010, No. 01, pp. 10-13.
3.Feasibility study on production and preparation of sodium citrate_ Gou Jibin.[4]Urwin C. S., Snow R. J. & Condo D. et al., "Factors Influencing Blood Alkalosis and Other Physiological Responses, Gastrointestinal Symptoms, and Exercise Performance Following Sodium Citrate Supplementation: A Review," International Journal of Sport Nutrition and Exercise Metabolism, Vol.31, No.2(2021), pp.168-186.
5.Scaravilli V., Di Girolamo L. & Scotti E. et al., "Effects of sodium citrate, citric acid and lactic acid on human blood coagulation," Perfusion, Vol.33, No.7(2018), pp.577-583.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium citrate manufacturers

US $1200.00-1100.00/ton2025-08-22
- CAS:
- 68-04-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00-0.00/KG2025-05-07
- CAS:
- 68-04-2
- Min. Order:
- 1KG
- Purity:
- 99.0-101%
- Supply Ability:
- 500kg/month