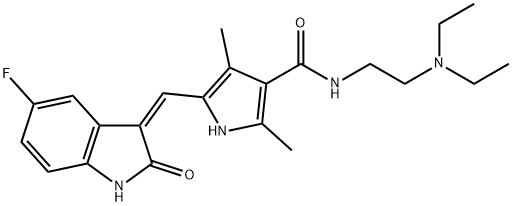
An oral multiple tyrosine kinase receptor inhibitor: Sunitinib
Description
Sunitinib is a yellow solid with the chemical formula C22H27FN4O2. This compound is Slightly soluble in Chloroform and Methanol. Sunitinib is a member of pyrroles and a monocarboxylic acid amide. Based on its good anti-tumor properties, it is widely used in the treatment of various cancers.
Use
Sunitinib was first approved by the U.S. FDA in January 2006 for advanced renal cell carcinoma, also as a second-line therapy for patients whose gastrointestinal stromal tumor is resistant to imatinib. This drug has a role as an angiogenesis inhibitor, an antineoplastic agent, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, a vascular endothelial growth factor receptor antagonist, an immunomodulator, and a neuroprotective agent. Currently, Sunitinib is used to treat gastrointestinal stromal tumors (GIST; a type of tumor that grows in the stomach, intestine (bowel), or esophagus (tube that connects the throat with the stomach) in people with tumors that were not treated successfully with imatinib (Gleevec) or people who cannot take imatinib[1]. Sunitinib is also used to treat advanced renal cell carcinoma (RCC, a type of cancer that begins in the cells of the kidneys).
Mechanism of action
Sunitinib malate is an oral receptor TKI of VEGF receptor 1 (VEGFR‐1), VEGFR‐2, VEGFR‐3, PDGF receptor‐α(PDGFR‐α), PDGFR‐β, and other receptor tyrosine kinases. Sunitinib inhibits cellular signaling by targeting multiple receptor tyrosine kinases (RTKs). These include all receptors for platelet-derived growth factors (PDGF-Rs) and vascular endothelial growth factor receptors (VEGFRs), which play a role in both tumor angiogenesis and tumor cell proliferation. The simultaneous inhibition of these targets therefore reduces tumor vascularization and triggers cancer cell apoptosis and thus results in tumor shrinkage.

Side effects
TKI-induced hypothyroidism (i.e., deficiency of thyroid hormone) is characterized by several symptoms, such as tiredness, poor ability to tolerate colds, and weight gain[2]. Sunitinib, for instance, is suspected of causing thyroid dysfunction more often than other TKIs. The most common adverse events associated with sunitinib therapy are fatigue, diarrhea, nausea, anorexia, hypertension, yellow skin discoloration, hand-foot skin reaction, and stomatitis. It is usually well tolerated and nephrotoxicity is very rare.
References
[1] Motzer R, et al. Sunitinib: Ten Years of Successful Clinical Use and Study in Advanced Renal Cell Carcinoma. The Oncologist, 2016; 22: 41–52.
[2] Shu M, et al. Hypothyroidism Side Effect in Patients Treated with Sunitinib or Sorafenib: Clinical and Structural Analyses. PLOS ONE, 2016.
You may like
Related articles And Qustion
See also
Lastest Price from Sunitinib manufacturers

US $0.00/g2025-04-21
- CAS:
- 557795-19-4
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000G
US $1.00-0.50/kg2025-04-21
- CAS:
- 557795-19-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200