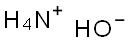Ammonium hydroxide-Health Hazard and Toxicity
Description
Ammonium hydroxide (NH4OH) is a solution of ammonia in water. Commonly called ammonia or ammonia water, the chemical is used as a cleanser and in manufacturing plastics, rubber, fertilizer and textiles.It's a colorless, clear liquid with a pungent-to-faint ammonia odor. Stable under normal temperatures and pressures, heating it produces ammonia gas. The chemical is incompatible with acids, hypochlorites, halogens, sodium hydroxide, galvanized surfaces and strong oxidizing agents, plus metals and their alloys.

Health Hazard
Ammonium hydroxide solutions are alkaline solutions, meaning they have high pH level. As a result, ammonium hydroxide is a severe eye, skin, and respiratory tract irritant, and readily burns tissue with which it comes in contact. Splashes to the eye may be serious, as contact may cause severe burns, irritation pain and possibly blindness. Direct contact with skin may cause severe burns if the chemical is not quickly rinsed away with copious amounts of water. Inhaling mists of ammonium hydroxide may result in irritation of the nose and throat with symptoms including burning, coughing, choking and pain. Inhaling concentrated mist may result in pulmonary edema and shock. Ingesting ammonium hydroxide may cause pain and burns of the esophagus and gastrointestinal tract.
Toxicity
Ammonia solutions are extremely corrosive and irritating to the skin, eyes, andmucous membranes. Exposure by inhalation can cause irritation of the nose, throat,and mucous membranes. Exposure to high concentrations of ammonia vapor (above approximately 2500 ppm) is life threatening, causing severe damage to the respiratory tract and resulting in bronchitis, chemical pneumonitis, and pulmonary edema, which can be fatal. Eye contact with ammonia vapor is severely irritating, and exposure of the eyes to ammonium hydroxide can result in serious damage and may cause permanent eye injury and blindness. Skin contact can result in severe irritation and burns; contact with the liquid results in cryogenic burns as well.
Ingestion of ammonium hydroxide burns the mouth, throat, and gastrointestinal tract and can lead to severe abdominal pain, nausea, vomiting, and collapse.Ammonium hydroxide has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. Chronic exposure to ammonia can cause respiratory irritation and damage.
Flammability and Explosibility
Ammonia vapor is slightly flammable (NFPA rating = 1) and ignites only with difficulty. Ammonia forms explosive mixtures with air in the range 16 to 25%.Water, carbon dioxide, or dry chemical extinguishers should be used for ammonia fires.
Reactivity and Incompatibility
Highly explosive nitrogen halides will form in reactions with halogens, hypohalites, and similar compounds. Reaction with certain gold, mercury, and silver compounds may form explosive products. Violent reactions can occur with oxidizing agents such as chromium trioxide, hydrogen peroxide, nitric acid, chlorite, chlorate, and bromate salts. Exothermic and violent reactions may occur if concentrated ammonium hydroxide solution is mixed with strong acids, acidic metal and nonmetal halides, and oxyhalides.
Storage and Handling
All work with this substance should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact.Containers should be tightly sealed to prevent escape of vapor and should be stored in a cool area separate from halogens, acids, and oxidizers. Containers stored in warm locations may build up dangerous internal pressures of ammonia gas.
Accident
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If ammonium hydroxide is ingested, obtain medical attention immediately.If large amounts of ammonia are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, soak up ammonium hydroxide with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Alternatively,flood the spill with water to dilute the ammonia before cleanup. Boric, citric, and similar powdered acids are good granular neutralizing spill cleanup materials.
Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
In some localities, ammonium hydroxide may be disposed of down the drain after appropriate neutralization and dilution. In a fume hood, the concentrated solution should be diluted with water to about 4% concentration in a suitably large container,and neutralized with a nonoxidizing strong acid such as HCl. The resulting solution can be discharged to the sanitary sewer. If neutralization and drain disposal are not permitted, excess ammonium hydroxide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
Related articles And Qustion
Lastest Price from Ammonium hydroxide manufacturers
US $0.00/kg2025-04-15
- CAS:
- 1336-21-6
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $9.00/KG2024-10-11
- CAS:
- 1336-21-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton