Ammonium Citrate Tribasic: Role in Quantum Dot Technology and its Preparation Method
General Description
Ammonium citrate tribasic plays a crucial role in both the synthesis of nitrogen-fluorine-doped carbon quantum dots and in their practical applications, particularly in detecting hypochlorite ions. The preparation method of Ammonium citrate tribasic involves a controlled reaction between citric acid and ammonium bicarbonate, utilizing hydration promoting agents to enhance stability and reduce carbon dioxide emissions. This innovative approach not only improves product quality but also addresses environmental concerns. The resultant quantum dots exhibit excellent biocompatibility and low cytotoxicity, paving the way for advancements in analytical chemistry and environmental monitoring, thereby showcasing the multifaceted benefits of ammonium citrate tribasic in modern applications.

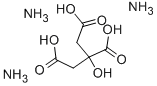
Figure 1. Ammonium citrate tribasic
Role in Quantum Dot Technology
Ammonium citrate tribasic plays a vital role in the synthesis of nitrogen-fluorine-doped carbon quantum dots, which are significant in the detection of hypochlorite ions. In the context of creating these quantum dots, ammonium citrate tribasic serves as a crucial precursor when combined with L-alanine and ammonium fluoride. The hydrothermal synthesis process facilitated by ammonium citrate tribasic results in quantum dots characterized by their strong blue fluorescence and small particle size. The unique properties imparted by ammonium citrate tribasic not only enhance the quantum yield but also improve the solubility of the resultant quantum dots, making them suitable for various analytical applications. 1
Enhancing Detection Capabilities
The use of ammonium citrate tribasic in the creation of nitrogen-fluorine-doped carbon quantum dots greatly contributes to the sensitive detection of hypochlorite ions in environmental and biological samples. The research indicates that these quantum dots can achieve a wide detection range, from 0 to 600 micromolar, with a notably low detection limit of 0.75 micromolar. This capability is largely due to the chemical characteristics of ammonium citrate tribasic, which aids in the formation of stable and high-quality quantum dots. The excellent performance of these fluorescent composites underscores the significance of ammonium citrate tribasic in developing new methodologies for monitoring hypochlorite ions, which can have profound implications in understanding various biochemical processes. 1
Biocompatibility and Practical Applications in Quantum Dots
In addition to their synthesis advantages, ammonium citrate tribasic-enhanced nitrogen-fluorine-doped carbon quantum dots exhibit exceptional biocompatibility and low cytotoxicity, making them suitable for use in living organisms. The ability of these quantum dots to detect hypochlorite ions in RAW 264.7 cells and environmental water samples demonstrates their practical applications in biochemistry and environmental science. The integration of ammonium citrate tribasic in this context confirms its importance not just as a reagent in synthesis, but also as a facilitator in advancing detection technologies. The research highlights the potential of using ammonium citrate tribasic-derived quantum dots in diagnosing diseases or monitoring environmental pollutants, paving the way for further innovations in analytical chemistry and biochemistry. 1
Preparation Method
The preparation method for ammonium citrate tribasic involves a carefully controlled chemical reaction between citric acid and sodium bicarbonate. To begin, the citric acid solution is maintained at a temperature not exceeding 10 degrees Celsius and subjected to a pressure greater than 4.5 megapascals. During this process, ammonium bicarbonate is added to the citric acid solution, resulting in a reaction that produces ammonium citrate tribasic. This reaction is further enhanced by employing a hydration promoting agent that helps stabilize the formation of the product while capturing byproducts such as carbon dioxide. The resultant water phase is subsequently collected, concentrated, and crystallized to obtain the final product, ammonium citrate tribasic. 2
Advantages
The preparation method of ammonium citrate tribasic not only yields the desired compound but also addresses environmental concerns linked to carbon dioxide emissions. By utilizing a hydration promoting agent such as tetrahydrofuran or cyclohexane, the process effectively captures carbon dioxide in solid form, significantly reducing harmful gas emissions. The choice of these agents furthermore prevents carbon dioxide from escaping during the crystallization phase, thereby enhancing efficiency. Throughout the reaction, stirring at controlled speeds between 400 to 500 revolutions per minute facilitates a more rapid and effective transformation of the reactants into ammonium citrate tribasic. Ultimately, this innovative method not only guarantees a high-quality product but also promotes sustainability in its manufacturing process. 2
References:
[1] QIANCHUN ZHANG. Preparation of One-Emission Nitrogen-Fluorine-Doped Carbon Quantum Dots and Their Applications in Environmental Water Samples and Living Cells for ClO- Detection and Imaging.[J]. Journal of Analytical Methods in Chemistry, 2023, 2023. DOI:10.1155/2023/7515979.Lastest Price from Ammonium citrate tribasic manufacturers

US $1200.00-1100.00/ton2025-08-08
- CAS:
- 3458-72-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $100.00-75.00/kg2025-04-21
- CAS:
- 3458-72-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton