Zinc Trifluoromethanesulfonate: Applications in Battery Technology and its Toxicity
General Description
Zinc trifluoromethanesulfonate is integral to advancing battery technologies, particularly in zinc-ion batteries, due to its ability to enhance hydrogel electrolytes with exceptional ionic conductivity and stability. This compound also plays a critical role in aqueous rechargeable zinc-ion batteries by preventing zinc dendrite formation and mitigating anode corrosion, thereby improving performance and longevity. While zinc trifluoromethanesulfonate offers significant benefits, potential health risks exist, including nutritional imbalances and gastrointestinal damage from excessive exposure. Chronic exposure can lead to symptoms such as anemia and respiratory issues, necessitating careful handling to ensure safety in its applications.

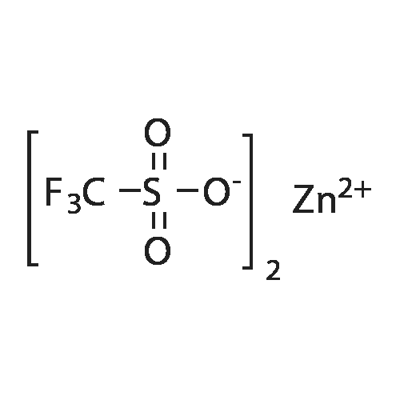
Figure 1. Zinc trifluoromethanesulfonate
Applications in Battery Technology
Zinc trifluoromethanesulfonate plays a crucial role in developing advanced battery technologies, particularly in the context of zinc-ion batteries (ZIBs). The unique properties of zinc trifluoromethanesulfonate facilitate the production of hydrogel electrolytes that significantly enhance the performance of these batteries. By incorporating zinc trifluoromethanesulfonate into a poly(vinyl alcohol) matrix, researchers have successfully created a hydrogel electrolyte with exceptional ionic conductivity and stability. These properties are vital for ensuring efficient energy transfer during charging and discharging cycles, whereby the incorporation of zinc trifluoromethanesulfonate contributes to improved electrochemical performance. The innovative self-healing capabilities of the hydrogel, owing to hydrogen bonding, further amplify the durability of zinc-ion batteries, adding a layer of sustainability to battery technology by reducing potential electronic waste. 1
Enhancing Performance Through Aqueous Zinc-Ion Batteries
In the realm of aqueous rechargeable zinc-ion batteries (ARZIBs), zinc trifluoromethanesulfonate serves as a fundamental component that addresses critical obstacles, such as zinc dendrite formation and the corrosion of zinc metal anodes. These challenges have historically impeded the performance and longevity of zinc-based batteries. By coating a polypropylene separator with zinc trifluoromethanesulfonate and poly(vinylidene fluoride-hexafluoropropylene), researchers have developed a novel approach that minimizes the adverse reactions associated with zinc anodes in aqueous solutions. As a result, this innovative separator not only mitigates zinc dendrite growth but also prolongs the operational life of zinc metal anodes, showcasing the efficacy of zinc trifluoromethanesulfonate in enhancing battery performance. Consequently, zinc-ion batteries equipped with this technology exhibit remarkable cyclic stability, significantly improving their appeal for large-scale energy storage applications. 2
Future Prospects and Applications
The incorporation of zinc trifluoromethanesulfonate in battery systems indicates a forward-thinking approach to energy storage technology, paving the way for the development of more reliable and efficient batteries. The self-healing properties of components containing zinc trifluoromethanesulfonate offer promising possibilities in maintaining battery performance over prolonged usage. For instance, the ability of zinc-ion batteries to recover from operational damage positions them as a viable option for sustainable energy solutions. Ongoing research into modifying battery components further highlights the versatility of zinc trifluoromethanesulfonate, as it can be tailored for different application requirements. As battery technology continues to evolve, zinc trifluoromethanesulfonate will undoubtedly remain a prominent focus, driving advancements toward safer, more efficient, and environmentally friendly energy storage systems. The integration of this compound in future battery designs will likely facilitate innovations aimed at addressing both performance standards and sustainability goals within the industry.
Toxicity
Zinc trifluoromethanesulfonate can pose various health risks when exposure is not adequately managed. One primary concern is that excessive absorption of zinc can lead to nutritional imbalances, particularly affecting the absorption of copper and iron, which could result in anemia. The competitive binding of these essential minerals may cause negative health implications, particularly in conditions like amyotrophic lateral sclerosis, where unbalanced levels of zinc, specifically zinc trifluoromethanesulfonate, may interfere with normal physiological functions. Furthermore, when zinc trifluoromethanesulfonate undergoes dissolution in stomach acid, it can produce corrosive byproducts such as zinc chloride, which may damage the stomach lining. Therefore, proper handling and usage of zinc trifluoromethanesulfonate are essential in preventing such adverse health effects. 2
Health Effects and Symptoms
Chronic exposure to zinc trifluoromethanesulfonate can lead to symptoms that are detrimental to overall health, such as anemia, lethargy, and gastrointestinal disturbances. Individuals may experience ataxia and a reduction in beneficial cholesterol levels, potentially increasing the risk of cardiovascular issues. Additionally, there is evidence suggesting that prolonged exposure to high levels of zinc may cause pancreatic and reproductive damage. Acute exposure, especially via ingestion, can result in symptoms such as stomach cramps, nausea, and vomiting, while inhalation of zinc compounds can lead to metal fume fever, characterized by chills, fever, and respiratory distress. Contact with zinc trifluoromethanesulfonate can also cause skin irritation, highlighting the importance of safe handling practices to mitigate these risks. 2
References:
[1] LI C, WANG W, LIU S, et al. Adjusting zinc deposition behaviors by a modified separator to acquire zinc anodes for aqueous rechargeable zinc-ion batteries?[J]. Dalton Transactions, 2023, 36: Page 12567 to 13012. DOI:10.1039/D3DT02212A.[2] DR. SHUO HUANG. A Self-Healing Integrated All-in-One Zinc-Ion Battery[J]. Angewandte Chemie, 2019, 131 13: 4109-4456. DOI:10.1002/ange.201814653.
You may like
See also
Lastest Price from Zinc trifluoromethanesulfonate manufacturers

US $2.00-5.00/KG2025-07-10
- CAS:
- 54010-75-2
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons

US $10.00/KG2025-04-21
- CAS:
- 54010-75-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt