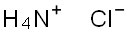Ammonium chloride
Ammonium chloride, NH4Cl, is a colorless cubic crystal or powder. It tastes cool, sublimates or decomposes when heated. In wet and rainy weather, it absorbs moisture and agglomerates. It is easily soluble in water, soluble in glycerin, slightly soluble in alcohol, and insoluble In acetone and ether. It can be divided into pharmaceutical grade ammonium chloride, industrial grade ammonium chloride, agricultural grade ammonium chloride, and reagent grade ammonium chloride.

Application
Medicinal grade ammonium chloride is an expectorant, antitussive, and diuretic; industrial ammonium chloride is mainly used in dry batteries, batteries, ammonium salts, tanning, electroplating, medicine, photography, electrodes, adhesives, etc.; agricultural grade chlorine Ammonium Chloride Agriculture Ammonium chloride is a nitrogen fertilizer with a nitrogen content of more than 25%. It is suitable for neutral and calcareous soils. It has a significant effect on increasing the yield of rice, corn, wheat, cotton, millet, rape, hemp and other crops. ; Reagent grade ammonium chloride is used for metal welding, electroplating, leather tanning and dry battery production.
Purification method
A method for improving the quality of industrial ammonium chloride products, the steps are as follows,
(1) First dissolve ammonium chloride into a saturated solution of ammonium chloride with water at 50°C, where the content of sulfate ion SO42 in ammonium chloride is about 0.05%;
(2) Cool down the saturated ammonium chloride solution to 12°C to precipitate ammonium chloride crystals;
(3) Pass the ammonium chloride crystal liquid through a thickener to increase the solid-liquid ratio. When the solid-liquid ratio reaches 16:84, enter the centrifuge for dehydration and drying to obtain the finished ammonium chloride, and the sulfate ion in the finished ammonium chloride The content of SO4 2- is about 0.01%;
(4) Add a barium chloride solution that is not excessive compared to the sulfate radicals in the mother liquor I to the mother liquor I thrown out during the dehydration of the centrifuge to generate barium sulfate precipitation and filter to obtain the filtered mother liquor II. The mother liquor II is heated After reaching 50°C, it is used to dissolve ammonium chloride and recycled.
The first step can be carried out in a hot liquid container. After it is fully dissolved into a saturated ammonium chloride solution, it is cooled by heat exchange through a heat exchanger, and then enters the cold liquid container to precipitate ammonium chloride crystals. In the fourth step, after raising the mother liquor II to 50°C, it is put into the hot liquid container of the first step to dissolve ammonium chloride and recycled.
You may like
Related articles And Qustion
See also
Lastest Price from Ammonium chloride manufacturers

US $1.00/g2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 12125-02-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt