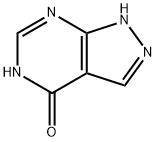
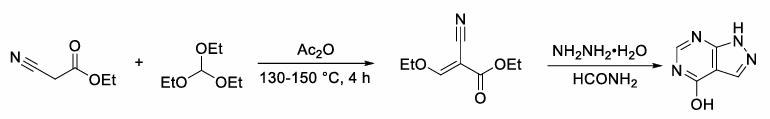
Allopurinol - Reaction / Application on synthetic works
Allopurinol is xanthine oxidase-activated prodrug of thymidine phosphorylase inhibitor. It is also an important organic intermediate to synthetize substituted pyrimidine products.
The following example is about its application on modifications of the thymidine phosphorylase inhibitor. [1]
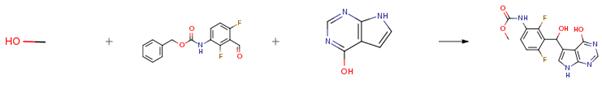
In a reaction vessel, 7H-pyrrolo[2,3-d]pyrimidin-4-ol (37, 855 mg, 6.33 mmol) is combined with (2,4-difluoro-3-formyl-phenyl)-carbamic acid benzyl ester (33, 2.03 g, 6.96 mmol) and potassium hydroxide (1.06 g, 19.0 mmol) and 5 mL of methanol is added. The resulting solution is stirred at 50 °C for 2 days. The reaction is diluted with hydrochloric acid and extracted with ethyl acetate, and the resulting solids collected by filtration and dried under vacuum. The organic layer is separated from the filtrate and the aqueous layer extracted twice more with ethyl acetate. The organic layers are combined and washed with water, brine, dried with magnesium sulfate and filtered. The filtrate is concentrated under vacuum and the resulting material is suspended in acetonitrile and sonicated for 1 hour. The solid is collected by filtration and dried under vacuum, then combined with the first solid collected to provide the desired compound (38, 2.05 g).
The following example is about its application on the synthesis of pyrrolopyriidine derivatives [2]

Into a 100ml single neck flask was added 3.375g (25mmol) 4- hydroxy-pyrrolo [2,3-d] pyrimidine, and then phosphorous oxychloride was slowly added 50 g (326.2mmol), and then was slowly warmed to 110 °C, with stirring at reflux 1h Thereafter, the reaction system by the suspension becomes dark supernatant. Reduced pressure after ice-cooling phosphorus oxychloride was evaporated to dryness, to the residue was added 15mL of water after, the ice bath was slowly added 10 percent NaOH aqueous solution was slowly adjusted to PH = 7-8, a large amount of brown solid precipitated, pumping filtered off, washed with water (10mL * 2) and dried. Wherein NaOH aqueous solution is not limited to, use in adjusting the PH can have KOH, CsOH is equally capable of adjusting pH of an aqueous solution of an alkaline substance. After to the dried solid was added 20mL 100mL EA and petroleum ether, and heated to 50 , 1h while stirring until substantially dissolved, 1.2g of activated carbon was added and heating continued for 30 minutes followed by filtration the organic phase under reduced pressure after dried and concentrated to give a yellow solid, which was with ethyl acetate / petroleum ether = 1/3 mixed solvent (20 mL) were successively beating, suction filtration, washed with petroleum ether, and dried to give 3.5g (theoretical yield 3.84 g) as a pale yellow solid, yield 91.5 percent.
The following example is about its application on the synthesis of substituted pyrimidine products [3]

To a solution of 1.0 g of 4-hydroxypyrrolo[2,3-d]pyrimidine in 20 ml of DMF, 3.8 g (2.5 eq) of bis(trimethylsilyl)acetamide was added, and the resulting solution was stirred at 40° C. in an oil bath for about two hours. Completeness of silylation was indicated by NMR analysis of an aliquot showing disappearance of the N-3 proton signal. The reaction was cooled to ambient temperature and 1.6 g (1.2 eq) of N-bromosuccinimide (NBS), was added in one portion. The reaction mixture was protected from light and stirred at ambient temperature until completion was indicated by NMR analysis (disappearance of pyrrole C-H doublets and emergence of a single, finely split doublet at δ7.16, about 2 h). The mixture was poured into 50 mL of water, with stirring. After 1-2 hours, the product was collected by filtration, washed with water, and dried. Filtration and drying gave 1.2 g of 4-hydroxy-5-bromopyrrolo-[2, 3-d]pyrimidine (75 percent yield), mp 269-271° C.
References
1.Plexxikon I, Ibrahim PN, Spevak W, Cho H, Shi S, Zhang C, Zhang Y. Compounds and methods for kinase modulation, and indications therefor. WO2011/63159[P], 2011, A1, Page column 189-190.
2.Shanghai Ya Ben Chemical Co. Ltd. Lin Z, Xu J, Jiang Y. A 4-chlorotrifluoro methylbenzene Pyrrolopyriidines and method for the preparation of (by machine translation). CN105622616[P], 2016, A, Paragraph 0053; 0054; 0055.
3.Eli Lilly and Company. Process for the synthesis of 4-hydroxy-5-halopyrrold [2,3-d]pyrimidine intermediates. US5235053[P], 1993, A.
You may like
Related articles And Qustion
Lastest Price from Allopurinol manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 315-30-0
- Min. Order:
- 1KG
- Purity:
- 98%-102%;USP
- Supply Ability:
- 10 TONS

US $10.00/KG2025-04-21
- CAS:
- 315-30-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt