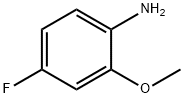
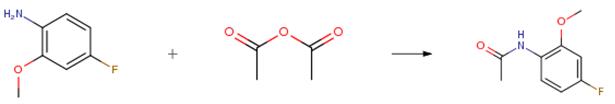
4-fluoro-2-methoxyaniline - Reaction / Application on synthetic works
4-Fluoro-2-methoxyaniline is a useful synthetic intermediate. It is used to prepare ortho-substituted phenylureas as 5-Hydroxytryptamine (5-HT3) receptor antagonists. It is also used to synthesize hydroxamic acids and their prodrugs as inhibitors for Botulinum neurotoxin A light chain.
It is also an important organic intermediate to synthetize substituted benzene products.
The following example is about its application on the synthesis of a key intermediate of AZD7594 [1]
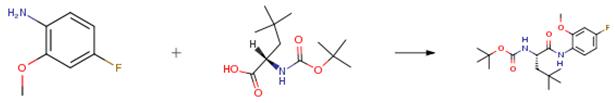
To a solution of EDCI•HCl (858.8 mg, 4.48 mmol) in DMF (10 mL) and CH2Cl2 (10 mL) were successively added OxymaPure (637.0 mg, 4.48 mmol) at room temperature. After stirring the reaction mixture for 5 min, 4-fluoro-2-methoxyaniline (704.2 mg, 4.48 mmol) in DMF (1 mL) and DIPEA (882 μL, 6.11 mmol) were added, and the resulting mixture was stirred for 10 h at the same temperature. The volatiles were removed under reduced pressure. The residue was diluted with ethyl acetate and 1 N HCl aq. was added. The resulting mixture was extracted with ethyl acetate twice, the combined organic layers were washed with aqueous saturated NaHCO3 twice, H2O, brine and dried over Na2SO4. After filtration, the residue was concentrated under reduced pressure and the resulting residue was purified by silica gel chromatography (ethyl acetate/n-hexane = 8/92 to 66/34) to give the product (1.41 g, 3.83 mmol, 94percent) as a white solid.
The following example is about its application on the synthesis of pyrimidine derivatives. [2]
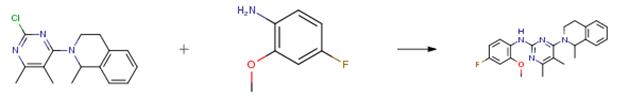
A reaction mixture of 4-fluoro-2-methoxy-aniline (0.155 g, 1.10 mmol), 5,6-dimethyl -2-chloro4-(1-methyl-1,2,3,4-tetrahydroisoquinolin-2-yl)pyrimidine(0.24 lg, 0.84 mmol), triethylamine(0.15 ml, 1.1 mmol) and propyleneglycol (2 ml) was heated to 140° C for 5 hours, cooled to a room temperature, diluted with dichloromethane(10 ml), and then washed with water. The separated organic layer was concentrated and the residual oil was purified by column chromatography (ethyl acetate/n-hexane=1/1) to give 0.247 g of the product. (Yield 75.2 percent).
The following example is about its application on the synthesis of benzothiazole derivatives [3]
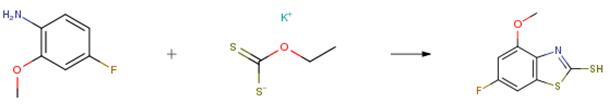
In a dry three-necked round-bottomed flask with a reflux condenser under normal temperature conditions, under nitrogen atmosphere, 14.11 g (0.10 mol) of 2-methoxy-4-fluoroaniline was added, and 200 ml of o-xylene was stirred and dissolved; then 4.81 g of sulfur (0.15 mol), 24.05 g (0.15 mol) of ethyl ethyl xanthate, 1.58 g (0.01 mol) of benzenesulfonic acid were added, and the temperature was raised to 100C for 4 hours; at room temperature, 4-dimethylaminopyridine is added to adjust the pH to neutral under high-speed stirring. Raise the temperature to 140°C for 2 hours, return to normal temperature, wash with water, and separate the organic solvent layer from the separatory funnel. Dry with anhydrous magnesium sulfate, filter and separate the desiccant, and evaporate o-xylene under reduced pressure to obtain crude 6-fluoro-4-substituted-benzothiazole-2-thiol product. The crude product was separated by column chromatography (300-400 mesh silica gel powder) by petroleum ether and ethyl acetate as eluents. Obtained 17.57 g of a slightly yellow powder of 6-fluoro-4-methoxybenzothiazole-2-thiol with a purity of greater than 99 percent. The isolated yield was 81.6 percent.
The following example is about its application on the synthesis of 4-fluoro-2-methoxy-5-nitroaniline [4]
In a dry round bottom flask acetic acid (950 ml) and 4-fluoro-2-methoxyaniline (380 gms) were added. The reaction mass was stirred at 25-30°C for 10-15 minutes. Acetic anhydride (439 gms) was added slowly to the reaction mass at 25- 35°C in 1.0-2.0 hrs. The reaction mass was heated to 90°C and stirred at same temperature for 3.0-5.0 hrs. The reaction mass was decomposed into water (1000 ml) and stirred at 25-30°C for 1.0-2.0 hrs. The solid was filtered and washed with water (300 ml). The reaction mass was extracted with ethyl acetate (2000 ml). Total organic layers were washed with NaHC03 solution (NaHC03 (100 gms) + water-4 (500 ml)) followed by washed with water (1000 ml), then washed with brine solution (NaCl (50 gms) + water-6(250 ml)) and dried over sodium sulphate (200 gms). The solvent was distilled out under vacuum. Petroleum ether (500 ml) was added to the residue and cooled to below 10°C and stirred for 30 minutes. The solid was filtered off and washed with petroleum ether (150 ml). The solid was dried at 50-60°C for 3-5 hrs (Yield - 410 gms; 83.13 percent).
References
1.Nonoyama A, Kumagai N, Shibasaki M. Asymmetric flow catalysis: Mix-and-go solid-phase Nd/Na catalyst for expeditious enantioselective access to a key intermediate of AZD7594[J]. Tetrahedron, 2017, 73(11):1517-1521.
2.Yuhan Corporation. Pyrimidine derivatives and processes for the preparation thereof. US6352993[P], 2002, B1, Page column 30.
3.South China Agricultural University. Zhou Z, Zhang Y, Jiang H, Yang X, Pan R, Wang B. A 2 - mercapto - 4 - substituted - 6 - fluorobenzonitrile benzothiazole and 2 - mercapto derivatives of nucleosides, application (by machine translation). CN107556266[P], 2018, A, Paragraph 0046-0047.
4.Aurobindo Pharma Limited. Chandiran T, Meenakshisunderam S, Sreenivasa Reddy M. A process for the preparation of 4-fluoro-2-methoxy-5-nitroaniline. WO2018/207120[P], 2018, A1, Page column 12.
You may like
See also
Lastest Price from 4-FLUORO-2-METHOXYANILINE manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 450-91-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $0.00/kg2025-03-06
- CAS:
- 450-91-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS