ALC-0315: Application and Synthesis
What is ALC-0315?
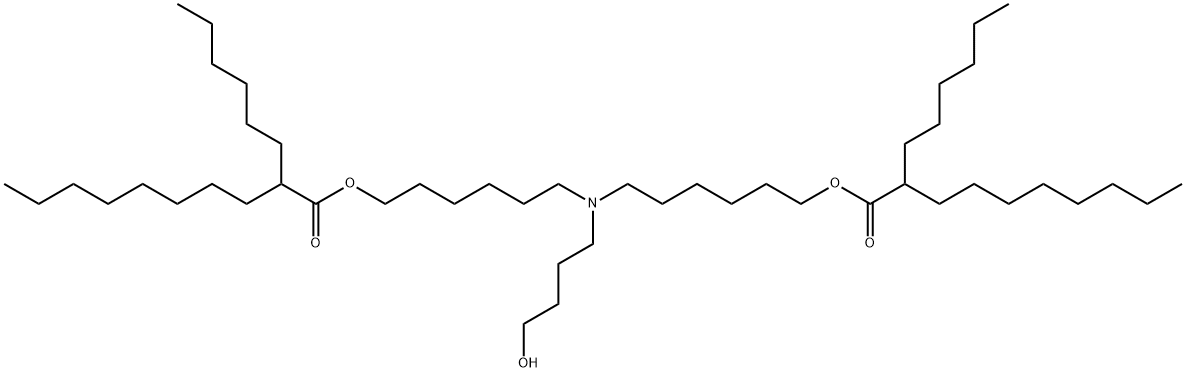
ALC-0315, also known as ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), is an ionisable amino lipid. It is a component of the lipid matrix of the prophylactic SARS-CoV-2 mRNA vaccine manufactured by Pfizer/BioNTech. This lipid ensures efficient vaccine assembly, protects mRNA from premature degradation, and facilitates the release of nucleic acids into the cytoplasm for further processing after endocytosis. It facilitates the cellular delivery of mRNA and its release into the cytoplasm through suspected endosomal destabilisation. It is commonly used as a lipid nanoparticle (LNP) delivery vehicle and has been used in studies of the mRNA COVID-19 vaccine.

Synthesis of ALC-0315
The first route of ALC-0315 synthesis (Fig. 1a) is covered by a patent. Step a1 is the acylation of an excess of hexanediol under the standard Steglich esterification conditions (condensation with dicyclohexylcarbodiimide in the presence of 4-dimethylaminopyridine as a catalyst). The reaction product is further used without purification. Step a2 is the oxidation with pyridinium chlorochromate; the product is extracted with hexane or petroleum ether, separated by chromatography on a silica gel column in the hexane–methylene chloride system. The final step a3 is the reductive amination. Sodium triacetoxyborohydride is the key reagent in this reaction; the reagent is commercially available or can be obtained in situ in the reaction between sodium borohydride and glacial acetic acid in benzene or dimethylacetamide. The target product ALC-0315 is obtained by chromatography on a silica gel column using a methanol gradient in methylene chloride. The described synthesis route has two drawbacks: (1) oxidation and reduction of the end carbon atom (steps a2 and a3) are used consecutively resulting in the oxidation state of the carbon atom remaining the same as it was initially (+1), which indicates that oxidation and reduction may possibly be substituted by condensation; (2) a side product, an urea derivative, is produced at the first stage (step a1), i.e., Steglich esterification, which complicates the purification of the end product.
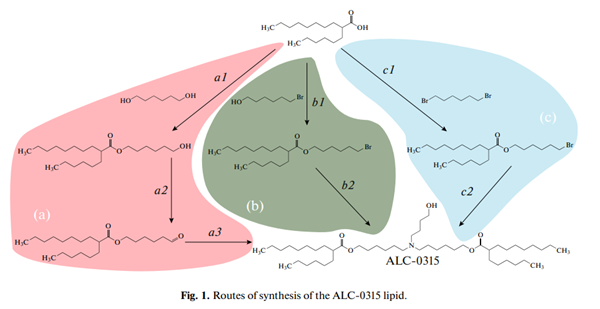
An alternative synthesis route (Fig. 1b) has been recently proposed in a patent. In this scheme, the oxidation–reduction problem was solved by using bromohexanol instead of hexanediol. This allowed us to reduce the number of stages. However, the esterification in the presence of a water-reducing agent remained. We propose further improvement of the ALC-0315 synthesis scheme (Fig. 1c) by using dibromohexane at the first stage. The simplicity of purification and the possibility to recycle dibromohexane, along with the use of K2CO3 as a condensing agent instead of the more expensive and hazardous carbodiimide derivatives make this synthesis route economically attractive.
We synthesised the ALC-0315 lipid in two steps (Fig. 1c). 2-Hexyldecanoic acid was reacted with a fourfold excess of 1,6-dibromohexane in DMF in the presence of potash. After three days of stirring the reaction mixture at room temperature, the condensation reaction product was isolated by chromatography on silica gel with 85% yield. Dibromohexane recovery rate was 52%. The target product ALC-0315 was obtained in the condensation reaction between 2-hexyldecanoic acid, 6-bromohexyl ester and 4-aminobutanol under the same mild conditions as in the previous step. With only a 10% excess of 6-bromohexyl-2- hexyl decanoate according to the stoichiometric ratio of the reagents, the yield of the target product after silica gel chromatography separation was 62%. In addition, the mixture of the target product with the monoalkylation product was obtained, which was added to 6-bromohexyl-2-hexyl decanoate during subsequent repeats of synthesis. Taking into account recycling and silica gel column regeneration (consecutive washing with isopropanol and chloroform) the yield of the target product reached 80%. The structure of the obtained target compound was confirmed by the 1 HNMR spectra.
References
[1] I. A. BOLDYREV. A Route to Synthesize Ionizable Lipid ALC-0315, a Key Component of the mRNA Vaccine Lipid Matrix[J]. Russian Journal of Bioorganic Chemistry, 2023. DOI:10.1134/S1068162023020061.