Adverse Reactions to Ornidazole: Liver Damage and Fixed Drug Reactions
General Description
Ornidazole, a commonly used antimicrobial agent for anaerobic infections, has been associated with two distinct adverse reactions. Firstly, it can lead to rare but potentially severe liver damage, presenting as cytolytic, cholestatic, or mixed hepatotoxicity, and in some cases triggering autoimmune hepatitis. Prompt recognition and withdrawal of the drug are crucial to prevent further harm, with corticosteroids possibly indicated in cases of autoimmune hepatitis. Secondly, ornidazole can induce fixed drug reactions on the skin or mucous membrane, characterized by erythematous patches with well-defined borders, typically recurring at the same location. This immune-mediated reaction involves memory T cells and may present with systemic symptoms. Early recognition and diagnosis are essential for appropriate management.

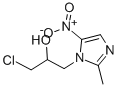
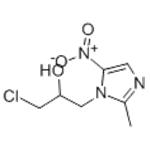
Figure 1. Ornidazole
Ornidazole-induced liver damage
Ornidazole-induced liver damage is a rare but potentially serious adverse reaction to the drug. Most drug-related injuries are mild and subclinical, but in rare cases, severe consequences and even death can occur. Drug reactions are divided into two types: type A reactions, which are predictable and related to the drug's pharmacologic action, and type B reactions, which are unpredictable and not dose-dependent. Hepatotoxins, including ornidazole, typically act through type B reactions. Drug-induced liver disease accounts for 10-50% of sporadic liver enzyme elevations in adults. While hepatic adverse effects from nitroimidazole derivatives like ornidazole are uncommon, a few cases have been reported previously. The characteristics of patients with ornidazole-induced liver damage include the absence of baseline liver abnormality, a temporal relationship to the use of the drug, and improvement upon withdrawal. Recurrence upon re-challenge further confirms the diagnosis. However, intentionally re-challenging patients is unethical. Liver injury caused by ornidazole can manifest as cytolytic, cholestatic, or mixed. Autoimmune hepatitis may also be triggered by the drug. Risk factors for drug-induced hepatitis include female gender, although this may be biased due to the more common indication of metronidazole or ornidazole use in women, such as vaginitis. It is important to recognize and withdraw the drug early to prevent further damage. In cases where ornidazole is suspected to be the cause of liver injury, other potential risk factors and underlying liver diseases should be investigated. Management of ornidazole-induced liver damage may involve corticosteroids, especially in cases where autoimmune hepatitis is present. In summary, ornidazole, a commonly used nitroimidazole derivative, can cause hepatotoxic damage resembling acute cholestatic hepatitis. Prompt recognition and discontinuation of the drug are crucial to prevent further harm. 1
Ornidazole-induced fixed drug reaction on sole
Ornidazole-induced fixed drug reaction on the sole is a specific type of drug reaction that occurs on the skin or mucous membrane and typically reoccurs at the same location. FDR is characterized by erythematous patches with well-defined borders, which can develop into bullae in severe cases. Ornidazole is an antimicrobial drug used to treat anaerobic infections, and while it is generally safe, there have been reported cases of ornidazole-induced FDR. The exact mechanism behind FDR is not fully understood, but it is believed to be a delayed immune reaction involving memory T cells. These cells provide specific immunity against infections and other antigenic stimuli, and they play a role in the inflammatory response seen in FDR. The most common drugs associated with FDR include NSAIDs, antibiotics, antineoplastics, and others. In diagnosing ornidazole-induced FDR, it is important to differentiate it from other conditions such as tinea pedis (fungal infection). Patch tests with the suspected drug, skin biopsy, or drug provocation tests can be performed for confirmation. Histopathological examination of the affected skin may reveal liquefaction in the basal layer, inflammatory cell infiltration, melanin incontinence, and edema in the papillary dermis. While FDR can occur in various parts of the body, it is most commonly seen in oral mucosa, limbs, genital area, and perianal area. In the case of ornidazole-induced FDR, previous reports have shown involvement of the oral mucosa, upper limbs, trunk, and lower extremities, including the sole. Multiple lesions may occur, and systemic symptoms such as fatigue, fever, joint pain, and nausea can accompany them. Awareness of the characteristics of ornidazole-induced FDR is essential, especially in patients taking multiple medications. Early recognition and diagnosis can help prevent further complications and guide appropriate management. As more cases are reported in the literature, it is becoming evident that ornidazole carries a risk for FDR, and healthcare professionals should be vigilant in monitoring for this adverse drug reaction. 2
Reference
1. Tabak F, Ozaras R, Erzin Y, Celik AF, Ozbay G, Senturk H. Ornidazole-induced liver damage: report of three cases and review of the literature. Liver Int. 2003;23(5):351-354.
2. Emre S, Ahsen H, Aktaş A. Ornidazole-induced fixed drug reaction on sole: case report and review of the literature. Cutan Ocul Toxicol. 2017;36(3):294-296.
You may like
Related articles And Qustion
See also
Lastest Price from Ornidazole manufacturers
US $0.00/kg2025-11-21
- CAS:
- 16773-42-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $1.00/kg2025-06-09
- CAS:
- 16773-42-5
- Min. Order:
- 10kg
- Purity:
- 99 %
- Supply Ability:
- 10000kg