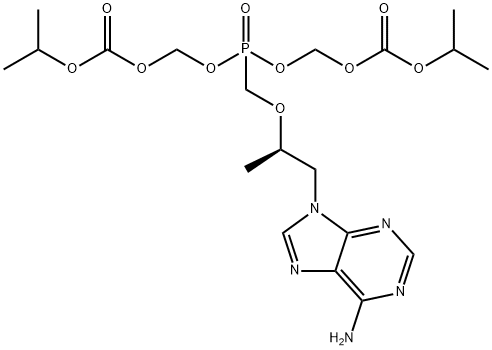
Adverse effects of Tenofovir
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS.
This product is used with other HIV medications to help control HIV infection. It helps to decrease the amount of HIV in your body so your immune system can work better. This lowers your chance of getting HIV complications (such as new infections, cancer) and improves your quality of life. This product is a combination of two different drugs: emtricitabine and tenofovir. Emtricitabine is called a nucleoside reverse transcriptase inhibitor, while tenofovir is called a nucleotide reverse transcriptase inhibitor. They are often called NRTIs.

This product should not be used by itself to help control HIV infection. Doing so can make your treatment less effective. Combination treatment with at least one other HIV medication should be used.
Side effects
Common side effects include nausea, rash, diarrhea, headache, pain, depression, and weakness. Severe side effects include high blood lactate and an enlarged liver. There are no absolute contraindications. It is often recommended during pregnancy and appears to be safe. It is a nucleotide reverse transcriptase inhibitor and works by decreasing the ability of the viruses to replicate.
Adverse effects
Tenofovir disoproxil is generally well tolerated with low discontinuation rates among the HIV and chronic hepatitis B population. There are no contraindications for use of this drug. The most commonly reported side effects due to use of tenofovir disoproxil were dizziness, nausea, and diarrhea. Other adverse effects include depression, sleep disturbances, headache, itching, rash, and fever. The US box warning cautions potential onset of lactic acidosis or liver damage due to use of tenofovir disoproxil.
Long term use of tenofovir disoproxil is associated with nephrotoxicity and bone loss. Presentation of nephrotoxicity can appear as Fanconi syndrome, acute kidney injury, or decline of glomerular filtration rate (GFR). Discontinuation of tenofovir disoproxil can potentially lead to reversal of renal impairment. Nephrotoxicity may be due to proximal tubules accumulation of Tenofovir disoproxil leading to elevated serum concentrations.
See also
Lastest Price from Tenofovir disoproxil manufacturers
US $0.00-0.00/kg2025-12-08
- CAS:
- 201341-05-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-07-14
- CAS:
- 201341-05-1
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 500kgs