ACP-105: Detection, Synthesis, Bioactivity
General description
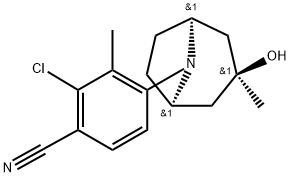
ACP-105 is an oral selective androgen receptor modulator (SARM) with pEC50 values of 9.0 and 9.4 for AR wild type and AR mutation T877A, respectively.
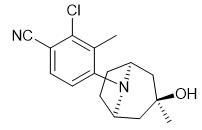
Fig. 1 The structure of ACP-105.
Synthetic routes
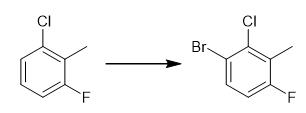
Fig. 2 The synthetic step 1 of ACP-105.
Stir 2-chloro-6-fluorotoluene (34.6 mmol) and iron (0.17 mmol) at room temperature. Add bromine (38.1 mmol) slowly in 3 portions at 1 minute intervals. Stir the reaction for additional 15 minutes. Add dichloromethane (50 mL). Transfer the reaction mixture to a separatory funnel. Wash with a sodium thiosulfate (solution (10%, 30 mL) until it is colorless. Wash the layers. Separate the layers. Saturate the organic layer with sodium hydrogen carbonate (30 mL). Dry over sodium sulfate. Filter the organic layer. Evaporate the organic layer. 1H NMR (CDCl3, 400 MHz) δ 7.53 (dd, J = 5.5, 8.6 Hz, 1H), 7.07 (t, J = 8.6 Hz, 1H), 2.35 (d, J = 2.3 Hz, 3H) [1].
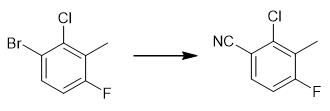
Fig. 3 The synthetic step 2 of ACP-105.
Charge a 3-bromo-2-chloro-6-fluorotoluene (0.78 mmol), zinc cyanide (0.78 mmol) and tetrakis (triphenylphosphine)palladium(0) (23 ?μmol) into a vial. Add DMF (1 mL) to the reaction mixture. Irradiate the mixture for 150 sec at 200 °C in a microwave oven. Add diethyl ether (30 mL) to the reaction mixture. Wash the reaction mixture with magnesium sulfate (4% solution, 3 x 20 mL) and brine (20 mL). Dry the organic layer over sodium sulfate. Filter the organic layer. Evaporate the organic layer. Purify the product by column chromatography on silica gel eluting with n-heptane/ethyl acetate (9:1). 1H NMR (CDCl3, 400 MHz) δ 7.43 (dd, J = 5.6, 8.8 Hz, 1H), 6.87 (t, J = 8.8 Hz, 1H), 2.36 (d, J = 2.4 Hz, 3H) [1].
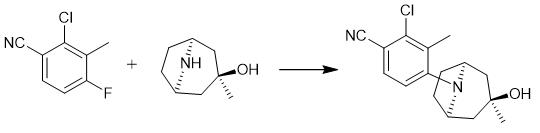
Fig. 4 The synthetic step 3 of ACP-105.
Dissolve 2-chloro-4-fluoro-3-methylbenzonitrile (14.6 mmol), endo-3-exomethyl-8-azabicyclo[3.2.1]octan-3-ol hydrochloride (15) (19.0 mmol) and K2CO3 (48.2 mmol) were d in dimethyl sulfoxide (40 mL). Stir the mixture under argon at 80 °C for 18 hours. Pour the reaction mixture into water (200 mL) and stir for 30 minutes. Filter off the off-white solid. Recrystallize twice from toluene. Evaporate the mother liquor. Recrystallize the residue. Prepare the hydrochloride salt by dissolving the product in diethyl ether. Add HCl to the reaction mixture (1 equivalent, 4M solution in 1,4-dioxane). Allow the mixture to stir for 15 minutes. Filter off the precipitated salt. Wash precipitated salt with diethyl ether. Dry precipitated salt. 1H NMR (CDCl3, 400 MHz) δ 7.39 (d, J=8.6 Hz, 1H), 6.84 (d, J=8.6 Hz, 1H), 3.82 (m, 2H), 2.36 (s, 3H), 2.32-2.22 (m, 2H), 2.17-1.98 (m, 2H), 1.92-1.77 (m, 4H), 1.26 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 155.8, 138.4, 132.0, 129.7, 117.9, 115.5, 105.1, 69.6, 59.3, 45.9, 34.7, 27.4, 17.9 [1].
Detection method
ACP-105 is a novel nonsteroidal selective androgen receptor modulator (SARM) with a tissue-specific agonist effect and does not have side effects associated with the use of common androgens. This research reports a comprehensive study for the detection of ACP-105 and its metabolites in racehorses after oral administration (in vivo) and postulating its structures using mass spectrometric techniques. To obtain the metabolic profile of ACP-105, a selective and reliable LC-MS/MS method was developed. The chemical structures of the metabolites were determined based on their fragmentation pattern, accurate mass, and retention time. Under the current experimental condition, a total of 19 metabolites were detected in ACP-105 drug administered equine urine samples. The study results suggest the following: (1) ACP-105 is prone to oxidation, which gives corresponding monohydroxylated, dihydroxylated, and trihydroxylated metabolites; (2) along with oxidation, there is a possibility of elimination of water molecule (dehydration) from the third position of the tropine moiety, resulting in the dehydrated analogs of corresponding monohydroxylated, dihydroxylated, and trihydroxylated metabolites; (3) from the study on the metabolites using LC-MS/MS, it is clear that the fragmentation pattern is identical and a great number of fragment ions are common in all the metabolites and the parent drug. (4) The ACP-105 and its metabolites were detected for up to 72 h; thus, the result is a valuable tool for evaluating its use and/or misuse in sport [2].
Bioactivity
Effects on rotorod performance and cued fear conditioning
Female mice are more susceptible to radiation-induced cognitive changes than male mice. Previously, we showed that, in female mice, androgens antagonize age-related cognitive decline in aged wild-type mice and androgens and selective androgen receptor modulators (SARMs) antagonize cognitive changes induced by human apolipoprotein E4, a risk factor for developing age-related cognitive decline. In this study, the potential effects of the SARM ACP-105 were assessed in female mice that were either sham-irradiated or irradiated with (137)Cesium at a dose of 10 Gy. Behavioral testing started 2 weeks following irradiation. Irradiation impaired sensorimotor function in vehicle-treated mice but not in ACP-105-treated mice. Irradiation impaired cued fear conditioning and ACP-105 enhanced fear conditioning in sham-irradiated and irradiated mice. When immunoreactivity for microtubule-associated protein 2 was assessed in the cortex of sham-irradiated mice, there was a brain area x ACP-105 interaction. While ACP-105 reduced MAP-2 immunoreactivity in the sensorimotor cortex, there was a trend towards increased MAP-2 immunoreactivity in the enthorhinal cortex. No effect on MAP-2 immunoreactivity was seen in the irradiated cortex or sham-irradiated or irradiated hippocampus. Thus, there are relatively early radiation-induced behavioral changes in female mice and reduced MAP-2 levels in the sensorimotor cortex following ACP-105 treatment might contribute to enhanced rotorod performance [3].
As an Extender Component
The aim of this study was to evaluate the effect of coconut water as a component of extender in different formulations for cooling equine sperm. One ejaculate of fourteen stallions was collected. Sperm was diluted to 50 x 10(6) sperm/mL using five different extenders: ACP-105: powdered coconut water extender (ACP-105, ACP Biotecnologia, Brazil); ACP-Milk: ACP-105 + 20 g/L of skimmed milk; ACP-EY 2.5%: ACP105 + 2.5% of egg yolk; ACP-EY 5%: ACP-105 + 5% of egg yolk; and BotuSemen (Botupharma, Botucatu, Brazil) and cooled in passive cooling device (BotuFlex, Botupharma, Botucatu, Brazil) at 5 and 15 degrees C for 24 hours. Sperm kinetics and plasma membrane integrity (PMI) were evaluated by computer-assisted sperm analysis and fluorescence staining, respectively, at TO (before cooling) and T24 (24 hours after cooling). Sperm kinetics did not differ at TO among groups (P > .05); however, at T24, these parameters were significantly lower in ACP-105 (5 degrees C, total motility [TM]: 9.2 +/- 3.6%; progressive motility [PM]: 2.7 +/- 1.6%; percentage of fast-moving spermatozo
Related articles And Qustion
See also
Lastest Price from ACP-105 manufacturers

US $1.00/g2025-04-21
- CAS:
- 899821-23-9
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 100kg

US $5.00/Box2025-04-21
- CAS:
- 899821-23-9
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes