Acetone Oxime: Properties, Applications, and Safe Handling in Industrial Chemistry
Introduction
Acetone oxime, chemically known as 2-propanone oxime, is an organic compound with the molecular formula C₃H₇NO. It is a versatile chemical that has garnered significant interest in various fields of chemistry and industrial applications. This compound is particularly notable for its role as an intermediate in chemical synthesis, especially in the production of pharmaceuticals, agrochemicals, and other fine chemicals. In this article, we will delve into the properties, composition, uses, and storage of acetone oxime, providing a comprehensive understanding of this important chemical compound.

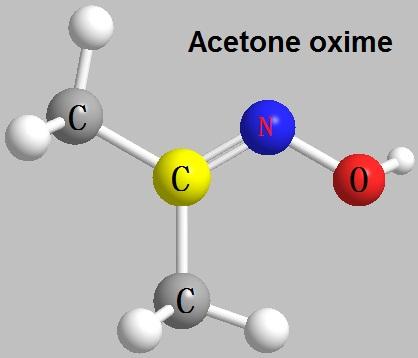
Figure 1 Characteristics of Acetone Oxime
Properties of Acetone Oxime
Acetone oxime is a colorless to pale yellow crystalline solid or liquid, depending on its purity and environmental conditions. It has a melting point of around 60°C and a boiling point of approximately 136°C. The compound is moderately soluble in water but is more soluble in organic solvents such as ethanol, ether, and chloroform. One of the most notable characteristics of acetone oxime is its ability to form stable complexes with metal ions, which has made it an important ligand in coordination chemistry.
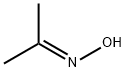
From a chemical perspective, acetone oxime is classified as a ketoxime, which means it is derived from a ketone (in this case, acetone) by the replacement of one oxygen atom in the carbonyl group with a nitrogen atom bonded to a hydroxyl group. This gives acetone oxime the general structure of (CH₃)₂C=NOH. The compound is known for its tautomeric equilibrium between the keto and oxime forms, which can influence its reactivity and interactions with other chemicals.
Main Components
Acetone oxime itself is the primary component of interest, but its synthesis and handling often involve other chemicals. The synthesis of acetone oxime typically involves the reaction of acetone with hydroxylamine hydrochloride in the presence of a base such as sodium hydroxide. This reaction yields acetone oxime and water as the main products. The purity of acetone oxime can vary depending on the specific method used for its synthesis and any subsequent purification steps.
In addition to the main oxime compound, acetone oxime preparations may contain small amounts of unreacted acetone, hydroxylamine, and other by-products from the synthesis process. These impurities can affect the physical properties and reactivity of the oxime, so careful purification and quality control are essential in industrial applications where high purity is required.
Applications of Acetone Oxime
Acetone oxime finds its application in various chemical processes, particularly in the production of other valuable compounds. One of its most important roles is as an intermediate in the synthesis of pharmaceuticals and agrochemicals. For example, it is used in the production of pesticides, herbicides, and fungicides, where it acts as a precursor to more complex molecules that possess biological activity.
In the pharmaceutical industry, acetone oxime is used to synthesize various active pharmaceutical ingredients (APIs). Its ability to form stable complexes with metals also makes it useful in coordination chemistry, where it can act as a ligand to stabilize metal centers in catalytic reactions or to form organometallic complexes.
Another significant application of acetone oxime is in the rubber industry, where it is used as an anti-scorching agent in the production of rubber and rubber-based products. This application takes advantage of the compound's ability to inhibit premature vulcanization, thereby extending the processing time of rubber mixtures and improving the quality of the final product.
Moreover, acetone oxime is also used as a solvent and stabilizer in the formulation of paints, coatings, and adhesives. Its chemical stability and compatibility with various other substances make it an ideal choice for these applications, where it can enhance the performance and shelf-life of the final products.
Storage and Handling
Proper storage and handling of acetone oxime are critical to ensure safety and maintain the compound's quality. Acetone oxime should be stored in a cool, dry, and well-ventilated area away from sources of ignition, heat, and incompatible materials such as strong oxidizing agents. It is typically stored in tightly sealed containers made of materials that do not react with the oxime, such as glass or certain types of plastic.
Due to its potential to decompose under certain conditions, acetone oxime should be kept in an environment where temperature fluctuations are minimized. Prolonged exposure to air and moisture can lead to the degradation of the oxime, resulting in the formation of by-products that could compromise its efficacy in industrial applications.
When handling acetone oxime, it is important to follow appropriate safety protocols to minimize the risk of exposure and chemical accidents. Personal protective equipment (PPE) such as gloves, goggles, and lab coats should be worn to prevent skin and eye contact. In case of a spill, the area should be ventilated, and the spill should be cleaned up using appropriate absorbent materials. Waste disposal should be conducted following local environmental regulations to avoid contamination and environmental harm.
Conclusion
Acetone oxime is a versatile and valuable compound with a wide range of applications in the chemical industry. Its unique properties, including its ability to form stable metal complexes and act as an intermediate in chemical synthesis, make it an essential component in the production of pharmaceuticals, agrochemicals, and rubber products. However, the handling and storage of acetone oxime require careful attention to ensure safety and maintain its chemical integrity. As research continues and new applications are discovered, acetone oxime is likely to remain an important chemical in various industrial processes.
[1] Harris W C, Bush S F. Vibrational Spectra and Structure of Acetone Oxime and Acetone Oxime‐O‐d[J]. The Journal of Chemical Physics, 1972, 56(12): 6147-6155.
[2] Liang X, Mi Z, Wang Y, et al. Synthesis of acetone oxime through acetone ammoximation over TS-1[J]. Reaction Kinetics and Catalysis Letters, 2004, 82: 333-337.
References:
[1] W. C. HARRIS S B. Vibrational Spectra and Structure of Acetone Oxime and Acetone Oxime-O-d[J]. Journal of Chemical Physics, 1972, 56 1: 6147-6155. DOI:10.1063/1.1677166.[2] XINHUA LIANG. Synthesis of acetone oxime through acetone ammoximation over TS-1[J]. Reaction Kinetics, Mechanisms and Catalysis, 2004, 82 2. DOI:10.1023/B:REAC.0000034845.65961.3e.
You may like
Related articles And Qustion
Lastest Price from Acetone oxime manufacturers
US $0.00-0.00/kg2025-11-14
- CAS:
- 127-06-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-08-29
- CAS:
- 127-06-0
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton