ABT-888: pharmacokinetic, mechanism of action and safety
General Description
ABT-888 is a potent PARP inhibitor that disrupts DNA repair, leading to synthetic lethality in cells with DNA repair deficiencies. It can enhance the cytotoxic effects of DNA-damaging agents and has shown promise in combination therapy for various malignancies. ABT-888 exhibits favorable pharmacokinetic properties, with good oral bioavailability and the ability to cross the blood-brain barrier. It primarily undergoes renal excretion and is influenced by renal clearance factors. The safety profile of ABT-888 is comparable to cytotoxic chemotherapeutic agents, with commonly reported adverse events including nausea, vomiting, and fatigue. Phase II trials have explored its tolerability in ovarian cancer treatment. Overall, ABT-888 offers a novel approach to cancer therapy through targeted inhibition of PARP.

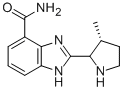
Figure 1. ABT-888
Pharmacokinetic
ABT-888, as studied in the phase 0 trial by Kummar et al, exhibits favorable pharmacokinetic properties. The drug demonstrates good oral bioavailability, with an absorption peak occurring within 0.5-1.5 hours and reaching a maximum concentration of 0.45 μM following a single 50 mg dose. It effectively inhibits PARP enzymes in cancer tissue and peripheral blood mononuclear cells within 3-6 hours, with complete activity observed within 24 hours. This suggests that twice-daily administration is suitable for maintaining persistent PARP activity inhibition. Additionally, ABT-888 is capable of crossing the blood-brain barrier. It primarily undergoes renal excretion, but approximately 13% of the drug undergoes hepatic metabolization via CYP2D6, resulting in the production of M8, a weak PARP inhibitor. Renal clearance is influenced by the presence of the OCT2 transporter in the kidney tubules, emphasizing the importance of adjusting the ABT-888 dose based on patient creatinine clearance. Notably, factors such as sex, age, BMI, and liver function do not significantly impact the drug's dosage, while exposure may be elevated in patients with CYP2D6 polymorphism or concurrent use of OCT2 inhibitors. 1
Mechanism of action
ABT-888 is a potent inhibitor of poly (ADP-ribose) polymerase (PARP), which plays a vital role in DNA repair. By targeting PARP, ABT-888 disrupts the repair of single-strand DNA breaks, leading to the accumulation of double-strand breaks during DNA replication. This ultimately results in synthetic lethality in cells with preexisting DNA repair deficiencies, such as those carrying BRCA mutations. When ABT-888 is administered in combination with DNA-damaging agents, such as chemotherapy or radiation therapy, it further impedes the DNA repair process, enhancing the cytotoxic effects on cancer cells. Additionally, ABT-888 has been shown to potentiate the anti-tumor activity of certain chemotherapeutic agents, making it a promising candidate for combination therapy in various malignancies. Through its targeted inhibition of PARP, ABT-888 demonstrates potential in the treatment of cancers with defective DNA repair mechanisms, offering a novel approach to cancer therapy. Further research and clinical trials continue to explore the full scope of ABT-888's mechanism of action and its therapeutic implications. 2
Safety
The safety profile of ABT-888, along with other PARP inhibitors, is comparable to that of cytotoxic chemotherapeutic agents. Commonly reported adverse events include nausea, vomiting, diarrhea, fatigue, headache, anemia, lymphopenia, and thrombocytopenia. When analyzing the adverse effects specific to individual PARP inhibitors, nausea and fatigue are frequently observed with olaparib, talazoparib, niraparib, and rucaparib. ABT-888 is associated with dizziness, nausea, and dysgeusia, while niraparib also causes anemia and thrombocytopenia. Rucaparib is linked to anemia. The tolerability and safety of ABT-888 in ovarian cancer treatment were explored in phase II trials, but noteworthy findings from a phase I study conducted by Metha et al should be considered. In this study involving 81 patients with solid tumors who underwent radiotherapy and received escalating doses of ABT-888 (10-300 mg orally twice a day), the most common toxicities included fatigue, nausea, loss of appetite, and vomiting. Grade 3-4 toxicities such as fatigue, hypokalemia, and hyponatremia were observed, with dose-limiting toxicities (DLTs) being hypokalemia and hyponatremia at 150 mg ABT-888 and one case of posterior reversible encephalopathy syndrome at 200 mg twice daily. At the highest dose (300 mg twice daily), five out of eight patients experienced nausea and/or vomiting. However, overall, the combination of ABT-888 with radiotherapy was well tolerated. 3
Reference
1. Bogliolo S, Cassani C, Dominoni M, Musacchi V, Venturini PL, Spinillo A, Ferrero S, Gardella B. Veliparib for the treatment of ovarian cancer. Expert Opin Investig Drugs. 2016;25(3):367-374.
2. George RR, Thomas R, Davice A, Mathew MS. Veliparib for the treatment of solid malignancies. J Oncol Pharm Pract. 2022 Jun;28(4):924-934.
3. Nomura H, Kataoka F, Aoki D, et al. Expression of potential biomarkers associated with homologous recombination repair in patients with ovarian or triple-negative breast cancer. Cancer Biomark, 2015.
Related articles And Qustion
See also
Lastest Price from ABT-888 manufacturers

US $0.00-0.00/kg2024-11-26
- CAS:
- 912445-05-7
- Min. Order:
- 1kg
- Purity:
- 99%, Single impurity<0.1
- Supply Ability:
- 1 ton

US $1.00/g2022-12-29
- CAS:
- 912445-05-7
- Min. Order:
- 10g
- Purity:
- 99.5%
- Supply Ability:
- 1000kg