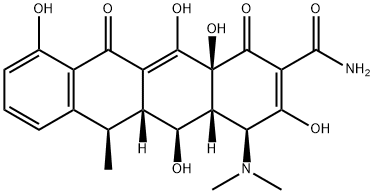
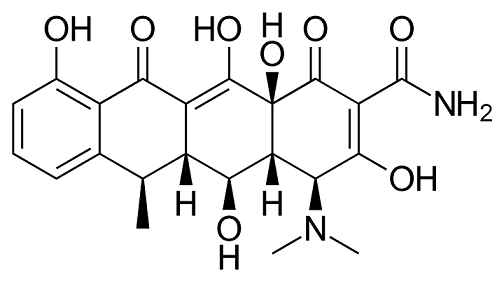
A second-generation tetracycline---Doxycycline
Doxycycline (alpha-6-deoxytetracycline) is a second-generation tetracycline with increased oral bioavailability and tissue penetration as a result of its improved lipophilicity compared with earlier tetracyclines. It is a semisynthetic derivative of oxytetracyclin and became available in 1967. It is available as both doxycycline monohydrate and doxycycline hyclate (Doryx, Doxy-100, Doxsig, Vibramycin, Zadorin).
Its mechanism of action is inhibition of microbial protein synthesis through interaction with 30S ribosomal subunits. It is almost universally administered orally, although also available intravenously, and has a prolonged serum half-life. As with the other tetracyclines, doxycycline has a wide spectrum of activity, but the development of widespread bacterial resistance and development of more bactericidal antibiotics has restricted its major uses to treatment of atypical, often intracellular, bacterial pathogens as well as malaria. Doxycycline is a crucial agent in the therapy of Q fever, brucellosis, melioidosis, atypical pneumonia, leptospirosis, and rickettsial infections. It is also an important agent for prophylaxis of many of the agents of biowarfare, such as anthrax.
Mechanism of Action
Tetracyclines, including doxycycline, inhibit bacterial protein synthesis through reversible binding to the ribosomal complex, preventing the association of aminoacyl-tRNA with the bacterial ribosome and interfering with protein synthesis. Access to Gram-negative bacterial ribosomal binding targets on the 30S subunit is through porins and via an energy-dependent process. Additional inhibition of protein synthesis occurs in mitochondria through binding to the 70S ribosomes. Although some doxycycline-susceptible parasites have mitochondria that are inhibited by this drug, these are not the only parasitic target. Apicoplast ribosomal subunits in P. falciparum appear to be inhibited by doxycycline. This process occurs late in the malarial cell cycle, explaining the slow antimalarial effect of doxycycline. The apicoplast houses enzymes involved in fatty acid synthesis and heme biosynthesis pathways.
Drug interactions
Doxycycline is a substrate of CYP3A4 enzymes and a moderate inhibitor of the same cytochrome P450 drug-metabolizing system. Therefore, its levels may be decreased by CYP3A4 inducers. Phenytoin, carbamazepine, and barbiturates apparently induce the metabolism of and reduce the serum concentration of doxycycline. Other drugs that can reduce doxycycline levels by this mechanism include nafcillin, nevirapine, and rifampicin. Cholestyramine binds doxycycline. Doxycycline has been shown to increase methotrexate levels in one patient, precipitating neutropenia and gut toxicity.
In patients treated for acne with both doxycycline and retinoic acid derivatives, the risk of benign intracranial hypertension is increased. Failure of the oral contraceptive pill (OCP) and increased risk of pregnancy has been suggested to be a consequence of doxycycline therapy. A pharmacokinetic study of women showed no reduction in serum ethinyl estradiol levels with concomitant doxycycline use. Furthermore, no progesterone rise was found, showing that breakthrough ovulation did not occur.
Related articles And Qustion
Lastest Price from Doxycycline manufacturers

US $0.00/kg2025-04-25
- CAS:
- 564-25-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $1.00/KG2025-04-21
- CAS:
- 564-25-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt