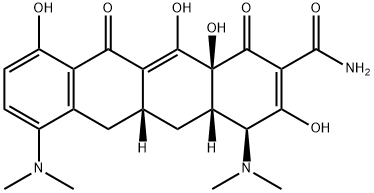
What is Minocycline?
The semisynthetic drug minocycline is a member of the tetracycline class and was discovered in 1972. The chemical name for minocycline is (2Z,4S,4aS,5aR,12aS)-2-(amino-hydroxymethylidene)-4,7-bis(dimethylamino)-10,11,12a-trihydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12- trione. Minocycline has an antimicrobial spectrum that is largely similar to tetracycline and doxycycline. The substitution changes the pharmacokinetic properties to a greater extent than its antibacterial features. Similar to other members of the tetracycline family, minocycline inhibits bacterial protein synthesis through its binding action on the bacterial 30S ribosomal subunits.
Minocycline is mainly marketed for oral administration, but a more soluble formulation of minocycline is available in some countries for parenteral administration. Minocycline is manufactured by several companies (under the brand names Dynacin, Minocin, Arestins, and Solodin, for example). Minocycline is used for a diminishing list of infections, because of increasing resistance and the availability of newer, more effective, and/or safer drugs.
Mechaniam OF Drug Action
Minocycline passes directly through the lipid bilayer or passively diffuses through porin channels in the bacterial membrane, but uptake across the cytoplasmic membrane is energy and pH dependent. Tetracyclines, like minocycline, bind principally to the 30S ribosomal subunits and specifically inhibit the enzyme binding of amino-acyl tRNA to the adjacent ribosomal acceptor site (A site) interfering with protein synthesis. The binding is reversible, providing an explanation of the bacteriostatic effect of the tetracyclines. Additionally, tetracyclines inhibit protein synthesis in mitochondria through binding to 70S ribosomes, which explains their antiparasitic activity in protozoa-containing mitochondria. A number of protozoa lacking mitochondria, nevertheless, are susceptible to tetracyclines. Tetracyclines may also cause alterations in the cytoplasmic membrane, thereby allowing leakage of nucleotides and other compounds from the cell. This action would explain the rapid inhibition of DNA replication (which occurs at a site on the membrane) that ensues when cells are exposed to concentrations of tetracycline in excess of that needed for protein inhibition. In addition, tetracyclines appear to inhibit adhesion of bacteria to human cells and so render the bacteria less pathogenic. These drugs probably inhibit the synthesis of a specific protein in the bacterial cell surface.
Clinical Uses
Tetracyclines are inexpensive antimicrobial agents, which have been used extensively in the prophylaxis and therapy of infections. Minocycline is used for a diminishing list of infections, because of increasing resistance and the availability of newer, more effective, and/ or safer drugs. Minocycline has been used in mycoplasma and chlamydial infections, and is as effective as tetracycline for the treatment of cholera, but it does not clear the feces of vibrios as rapidly as tetracycline . In vitro, a synergistic inhibitory effect on Vibrio cholerae was seen with cefazoline or cefotaxime in combination with minocycline. In chronic bronchitis and sinusitis due to pathogens sensitive to minocycline, the drug has the advantage of only requiring single- or twice-daily dosing and achieving high sputum concentrations. However, long-term use is contraindicated because of its vestibular side-effects, and doxycycline or tetracycline is preferred. Nowadays, minocycline has a very limited value for the treatment of surgical, biliary, bowel, and urinary tract infections. However, there are some specific indications for the drug.
See also
Lastest Price from Minocycline manufacturers

US $0.00/kg2025-04-25
- CAS:
- 10118-90-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 10118-90-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt