A promising photocathode material-Copper gallium selenide
I—III—VI2 chalcopyrite semiconductors have recently attracted considerable attention because of their potential applications for optoelectronic devices. Among them, CuGaSe2, one of the promising materials for solar cells, has been investigated by many researchers, and its bulk single crystals have been prepared by various methods such as melt growth, solution growth, and iodine transport. In the case of the iodine transport method, the resultant CuGaSe2 crystals were inevitably contaminated by the iodine agent. In addition, it was difficult to obtain a large single crystal[1].
In the case of melt growth, the CuGaSe2 chalcopyrite phase is known to be formed through a peritectic reaction from the (sphalerite#liquid) phase to the chalcopyrite phase. Therefore, a large CuGaSe2 single crystal is challenging to grow from the stoichiometric melt. The preparation method should be adopted at a temperature lower than the peritectic temperature to overcome this difficulty. For this purpose, a solution method has already been devised using several solvents such as In, CuI, Te, Pb, and Bi. However, in these cases, the solvents contaminated the grown crystals because of their good solubility in CuGaSe2. Thus, the solution method using a self-flux is recommended to obtain high-quality CuGaSe2 crystals.
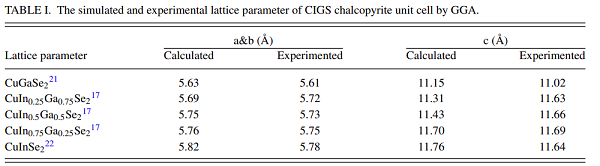
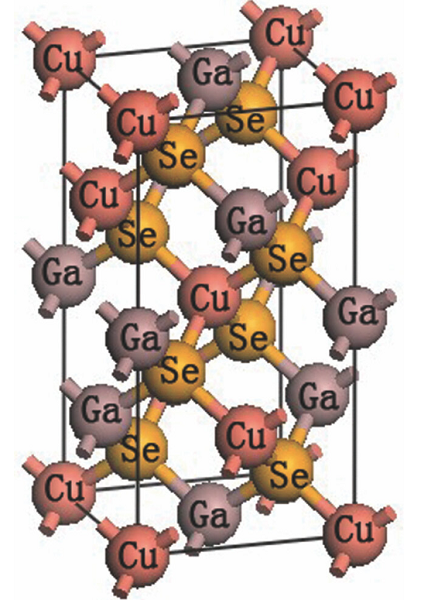
The copper chalcopyrite CuGaSe2 and the defect-related phase CuGa3Se5 are promising photocathode materials for solar hydrogen generation[2]. Existing devices exhibit photocurrents nearing 68% (CuGa3Se5)– 86% (CuGaSe2) of their theoretical limit, but they are plagued by photovoltage losses that reduce their energy conversion efficiency. The relaxed lattice constant of CuGaSe2 is a = 5.63 Å and c = 11.15 Å, which is reasonable to CGS, which is 0.35% and 1.17% larger than the experimental value a = 5.61 Å and c = 11.02 Å[3].
References
[1] H. Jitsukawa, T. Takizawa, H. Matsushita. “Phase diagrams of the (Cu2Se, CuSe)–CuGaSe2 system and the crystal growth of CuGaSe2 by the solution method.” Journal of Crystal Growth 186 1 (1998): 587–593.
[2] Ye Cheng. “Effect of charge selective contacts on the quasi Fermi level splitting of CuGa3Se5 thin film photocathodes for hydrogen evolution and methylviologen reduction.” EES catalysis 1 1 (2022).
[3] Xu-Dong Chen. “Hybrid density functional theory study of Cu(In1−xGax)Se2 band structure for solar cell application.” AIP Advances 4 1 (2014): 087118.