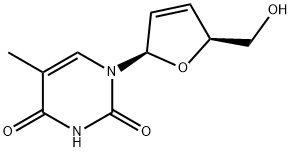
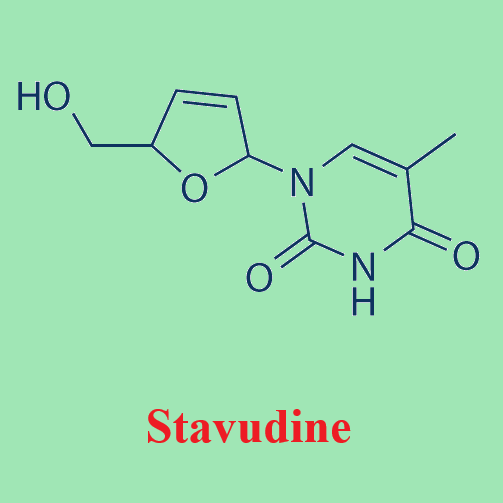
A potent inhibitor of HIV reverse transcriptase---Stavudine
The nucleoside analog stavudine (2u,3u-didehydro-2u3u-deoxythymidine, d4T) is a potent inhibitor of HIV reverse transcriptase in vitro. It is active against both HIV-1 and HIV-2. It was the fourth nucleoside reverse transcriptase inhibitor to become commercially available and is marketed under the trade name of ‘‘Zerit’’ by Bristol-Myers Squibb. Stavudine was first synthesized in 1966 by Horwitz and colleagues. There are a number of generic brands of stavudine, mostly manufactured in India as stavudine capsules. Several formulations were approved by the FDA for use in the USA in December 2008. There are also fixed-dose formulations of stavudine and lamivudine and fixed dose formulations of stavudine, lamivudine, and nevirapine. Pediatric formulations have also been developed and evaluated in generic fixed-dose combinations of stavudine, lamivudine, and nevirapine.
Mechanism of Drug Action
Stavudine, a synthetic pyrimidine (thymidine) analog, inhibits the replication of HIV following its phosphorylation in the cell to stavudine-5u-triphosphate. Stavudine triphosphate inhibits HIV reverse transcriptase by competing with endogenous deoxythymidine triphosphate, the natural substrate for the enzyme, as well as by causing premature HIV DNA chain-termination (due to the absence of a 3u hydroxyl group on the pentose ring necessary for 3u-5uphosphodiester linking), and thus inhibiting viral synthesis. Stavudine has a different phosphorylation pattern to zidovudine. Both drugs are sequentially phosphorylated by cellular enzymes to the mono-, di-, and triphosphate derivatives. However, stavudine is phosphorylated intracellularly to its 5u monophosphate derivative by the cellular thymidine kinase about 300- to 600-fold less efficiently than zidovudine, with a Km of 142 and 14 mM, respectively, in MT-4 cells. Stavudine does not accumulate in cells in its monophosphate form, again differing in this respect to zidovudine). There are similar levels of each of the phosphorylated derivatives of stavudine, with the initial phosphorylation step being rate limiting . However, because the ratelimiting step in zidovudine intracellular metabolism is the phosphorylation to the diphosphate form, there is accumulation of zidovudine monophosphate.
Bioavailability
Stavudine is rapidly absorbed after oral administration, with a mean bioavailability in adults following a 4 mg/kg oral dose, ranging from 70% to 90%. The mean oral bioavailability in HIV-infected pediatric patients is 70–80%. These values are consistent with the reported bioavailability of stavudine in mice and monkeys. The high stability of stavudine at low pH and minimal presystemic metabolism of stavudine may account for the excellent oral bioavailability. The oral bioavailability in children between seven months and 15 years of age ranges from 61% to 78% . Stavudine can be taken without regard to meals.
Drug interactions
Pharmacokinetic studies have shown no interaction between stavudine and a range of antimicrobials commonly used for the prevention of HIV-associated opportunistic infections . Methadone decreases gastrointestinal absorption of stavudine and consequently reduces AUC and Cmax. Simultaneous use of stavudine and didanosine is relatively contraindicated because of increased frequency of mitochondrial toxicity. Co-prescription of stavudine and ribavirin has been associated with an increased risk of mitochondrial toxicities, especially lactic acidosis. Co-prescription of agents known to cause peripheral neuropathy, particularly isoniazid and hydroxyurea, should be avoided because of their additive toxicity profiles. The use of stavudine is not recommended with didanosine, zalcitabine, or zidovudine.
Toxicity
Stavudine is generally well tolerated during short courses of therapy; however, significant mitochondrial toxicity, particularly peripheral neuropathy, is associated with prolonged use. Mitochondrial toxicities have been observed with all nucleoside reverse transcriptase inhibitors; however, stavudine has been observed to exhibit these especially frequently. This is most commonly believed to reflect nucleoside reverse transcriptase inhibitor-related inhibition of mitochondrial DNA (mtDNA) polymerase-g, an effect more evident with stavudine than with other nucleoside reverse transcriptase inhibitors. Inhibition of mtDNA polymerase-g results in defective cellular oxidative phosphorylation, which appears to be the basis for many of the clinical toxicities of stavudine therapy detailed below. Alternative or additional mechanisms may also contribute, particularly altered expression of various metabolic genes.
You may like
Related articles And Qustion
See also
Lastest Price from Stavudine manufacturers

US $5.00-0.50/KG2025-06-13
- CAS:
- 3056-17-5
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 3056-17-5
- Min. Order:
- 1KG
- Purity:
- 98%-102%; USP
- Supply Ability:
- 500KGS