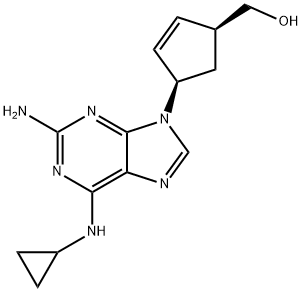
What is Abacavir sulfate?
Abacavir sulfate (also known as 1592U89) is a carbocyclic nucleotide analog that received US Food and Drug Administration (FDA) approval in 1998 for the treatment of HIV infections . Abacavir was developed from the compound carbovir (NSC 614846), by the addition of a 6-cyclopropylamino moiety at the 6-position of the purine ring. Although carbovir was initially found to have potent in vitro anti-HIV activity and was intensively researched as a potential antiretroviral drug target, its poor oral bioavailability in animal studies, unfavorable toxicology profile, and limited brain penetration precluded further clinical development. The 6-cyclopropylamino modifications to carbovir overcame these pharmacokinetic and toxicologic limitations while retaining its potent antiviral activity. Additionally, this modified carbovir compound also retained the improved in vivo stability conventionally associated with this structure compared with the other 2u,3u-dideoxynucleotides such as zidovudine, didanosine, zalcitabine, and stavudine.
MECHANISM OF DRUG ACTION
Abacavir is therapeutically classified as a nucleoside reverse transcriptase inhibitor (NRTI) and is the only 2u-deoxyguanosine nucleoside analog in this class. It is converted intracellularly through a unique mechanism to its active triphosphate form, which subsequently inhibits the HIV reverse transcriptase enzyme by competing with endogenous substrates of deoxyguanosine triphosphate (dGTP) for incorporation into the elongating proviral DNA chain of a replicating virus. Following this incorporation, the guanosine analog acts as a chain terminator and prematurely ceases DNA synthesis. Abacavir is manufactured as a hemisulfate salt and is available in tablet and oral liquid formulations under the trade name Ziagent, marketed by Glaxo SmithKline. Abacavir sulfate is also co-formulated with lamivudine [Kivexat (UK), Epzicomt (US)] and zidovudine and lamivudine (Trizivirt), both of which are available only as tablets.
TOXICITY
The initial studies of abacavir therapy showed association with a number of adverse effects. Many of these long-term studies included some adverse events seen in common with other nucleoside analog reverse transcriptase inhibitors. The incremental effect of addition of abacavir to a regimen and comparison with zidovudine with a similar background suggests that abacavir is associated with few specific adverse events seen with the drug combinations. Postmarket surveillance has reported a limited number of serious adverse events. Two serious adverse events are listed as a black box warning for abacavir. The adverse event that is most frequently limiting for abacavir therapy has been development of hypersensitivity reactions, which may be associated with fatal outcomes, particularly where there has been re-challenge. The other warning is for the potentially fatal outcome of hyperlactatemia and lactic acidosis.
CLINICAL USES
Abacavir is a potent NRTI which is useful as part of the backbone of combination antiretroviral regimens for HIV infection in both the developed and the developing world. The risk of hypesensitivity reactions associated with abacavir therapy is now uncommon with the use of HLA B5701 testing prior to prescribing abacavir. The D:A:D study exposed a potential risk of cardiovascular disease associated with abacavir use which is undergoing further investigation. The clinical use of abacavir in the management of HIV infection under various conditions is discussed. The use of the abacavir-lamivudine combination for treatment has recently been reviewed.
See also
Lastest Price from Abacavir manufacturers

US $0.00/G2025-04-21
- CAS:
- 136470-78-5
- Min. Order:
- 50G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month

US $60.00-40.00/KG2025-03-21
- CAS:
- 136470-78-5
- Min. Order:
- 1KG
- Purity:
- 99.99%
- Supply Ability:
- 200