A kind of preparation method and use of lactic acid
Background and overview
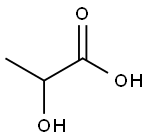
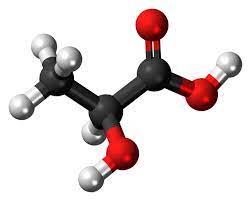
Lactic acid is also known as 2-hydroxypropionic acid and alpha-hydroxypropionic acid. The pure product is a colorless liquid, and the industrial product is a colorless to pale yellow liquid. Odorless and hygroscopic. Miscible with water, ethanol and glycerol, insoluble in chloroform, carbon disulfide and petroleum ether. Lactic acid is heated and decomposed under normal pressure, and when it is concentrated to 50%, part of it becomes lactic anhydride, so the product often contains 10% to 15% of lactic anhydride. Due to the presence of hydroxyl and carboxyl groups, esterification can occur under certain conditions. There is an asymmetric carbon atom in the molecular structure, forming two optical isomers d-body, l-body and racemate dl-body, a total of 3 optical isomers. D- and racemates are present in the meat tissue of humans and animals, while the levorotatory isomers are normal metabolites in mammals. It also occurs naturally in poppies, apples, tomato juice, and other fruits. Commercially available products, whether obtained by fermentation or synthesis, are racemates.

Preparation method【1】
Preparation of catalyst: Weigh AuCl3•HCl•4H2O (0.4072g) and Pd(NO3)2•2H2O (0.2635g) respectively to prepare 100mL precursor solution with molar concentration of 0.0099mol/L, add 4g CaF2Ca(PO4)2 after mixing(carrier);3000mg of P. haemorrhagum and 3000mg of dry cells of Bacillus licheniformis were added to the mixed solution of the precursor and the carrier, and then at room temperature, 200rpm was shaken and cultured for 48h, the above mixture was centrifuged, and the cells were washed The Au1Pd1-CaF2Ca(PO4)2 catalyst with a loading of 7.5% was obtained after three times of vacuum drying at 40°C.
Preparation of lactic acid: Dissolve 38.045g of 1,2-propanediol in 100 mL of water to obtain a solution of 5mol·L-1, which is mixed with 6.667g of AlCl3 (that is, the ratio of 1,2-propanediol to AlCl3 is 1:0.1), 2.1723 g catalyst (that is, the material ratio of metal to 1,2-propanediol is 1:500) is mixed and added to the high temperature and high pressure reaction kettle, oxygen is purging the air in the kettle and the reaction pressure is controlled by oxygenation; hour, then continue to heat up to 160 °C and maintain a stirring rate of 200 rpm, react for 2 hours and then cool down to room temperature, take out the material in the high temperature and high pressure reaction kettle and filter, the supported metal catalyst obtained by filtration is washed and dried with methanol and then recycled. The liquid was rectified to obtain lactic acid product, and the product was analyzed.
The conversion of 1,2-propanediol was 60% and the selectivity to lactic acid was 60% by analytical testing.
Use
Lactic acid is a versatile organic acid that can be used in brewing, medicine, leather, cigarettes, chemicals, food, printing and dyeing, etc. The distribution of lactic acid in Japan is roughly: about 20% for brewing, about 50% for food, about 10% for lactic acid derivatives, and about 20% for industrial uses such as leather. Lactic acid is widely used as acidulant, preservative and reducing agent in the food industry due to its mild and stable acidity, which helps to increase the flavor of food. It can be used in the production of refreshing beverages, candy, cakes, and in the processing and preservation of fish, meat and vegetables. Compared with edible acids such as citric acid and malic acid, it has strong competitiveness. Lactic acid is also an important sour agent and has a specific astringent sour taste. It can be used in all kinds of food and can be used in moderation according to production needs. It also has a strong bactericidal effect, which can prevent the growth of miscellaneous bacteria.
References
[1] Liu Ling, Shen Lingqin, Shao Shouyan, Xiong Xiong, Zhu Guisheng, Xu Baohua, Chen Gang, Huang Chunxia. A preparation method of lactic acid [P]. Jiangsu Province: CN113861020A, 2021-12-31.
Related articles And Qustion
See also
Lastest Price from Lactic acid manufacturers

US $1200.00-1100.00/ton2025-08-07
- CAS:
- 50-21-5
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $1.00/kg2025-06-26
- CAS:
- 50-21-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt