A Comprehensive Analysis of Magnolol
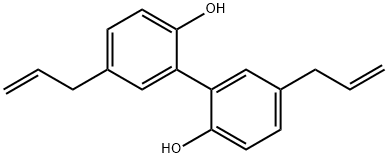
Magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl), a type of bisphenol compound, is a white fine powder with a spicy and fragrant smell. Honokiol is an analogue of it and its phenolic hydroxyl groups are placed in different relative locations. Magnolol is extracted and isolated from the root and branch barks of Magnolia officinalis Rehd. et Wils. It showed a variety of pharmacological properties, such as anticancer, antiinflammatory, antioxidant (Suh et al., 2017), antimicrobial, and neuroprotective activities. In addition, the value of magnolol was noticed as pesticidal agents in the field of agriculture. Over the past few decades, the total synthesis of it was accomplished by different methods. Although a possible biosynthetic pathway for it was proposed, and the relevant genes were preliminary investigated by bioinformatics, the complete biosynthetic pathway has not been validated so far.

Insights on the Multifunctional Activities of Magnolol
Magnolol (5,5'-diallyl-2,2'-dihydroxybiphenyl) is a polyphenolic binaphthalene compound and a structural isomer of honokiol. Both magnolol and honokiol are isolated from the stem bark of a traditional Chinese herbal medicine Magnolia officinalis, which has been used for management of nervous disturbance, abdominal distention or disorders, gastrointestinal food stagnancy, and coughing and dyspnea. It has showed a wide spectrum of beneficial activities, including anti-inflammation, antimicroorganism, antioxidation, antiangiogenesis, anticancer, neuroprotection, cardiovascular protection, and lipolysis activities. In this review article, the biological activities of it will be discussed comprehensively. It is often available for human in daily life because it is the major component of extract, which adds to mints and gums. It has been estimated that the safe dose of magnolol available for teenage is up to 1.64 mg/kg per day The release of LPS from Porphyromonas gingivalis has been involved in the inflammation-induced periodontitis development. In LPS-triggered RAW 264.7 macrophages, magnolol abrogates the inflammatory responses, as evidenced by downregulation of NF-κB signaling, activation of NF-E2-related factor 2 (Nrf-2)/heme oxygenase-1 (HO-1) signaling, and suppression of iNOS, COX-2, Prostaglandin E2 (PGE2), and NO expression. In addition, administration of p38 MAPK and reactive oxygen species (ROS) activators may reverse the effects of it.[1]
The extensive research indicates that magnolol undergoes glucuronidation (phase II metabolism) before its elimination in human and rats, as indicated by transferring glucuronic acid from UDP-glucuronic acid to this medicine and making it more soluble and excreted by the kidney. Such glucuronidation involves several UDP-glucuronosyltransferase (UGTs) isoforms, including UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A9, UGT1A10, and UGT2B7. Of which UGT2B7 is the major enzyme responsible for magnolol glucuronidation in human liver microsomes (HLM). In addition, UGT1A10 and UGT2B7 are the two major contributors in human intestine microsomes (HIM). Glucuronides of β-estradiol or SN-38 generated in microsomal or HeLa1A1 cell incubations have been quantified, and it shows inhibitory effects (β-estradiol: IC50 value of 36.8 μM and 22.6 μM, respectively; SN-38: IC50 value of 13.2 μM and 16.4 μM, respectively). These are available for the strategies in protecting neural and cardiovascular diseases. In addition, it mediates the expression of proliferation-related proteins, which might be the potential targets for managing cancers. Metabolism regulation and ion mediation are the two critical cellular physiological processes, during which imbalanced homeostasis may result in severe pathology and be restored by it. These findings have greatly increased the interest in bridging magnolol to the clinic as a promising therapeutic agent.
Magnolol and its semi-synthetic derivatives
Cancer is the uncontrolled growth of abnormal cells that can invade nearby and distant tissues and organs. It involves changes in cellular or tissue structure and function. Genetic mutations are the predominant cause of the growth of neoplastic cells, which tend to increase their reproductive lifespan. In this sense, Magnolol (MG) is a hydroxylated biphenyl compound derived from the bark (roots and branches) of Magnolia species such as M. officinalis, M. obovata, and M. grandiflora. Historically, the genus Magnolia represents plants of the family Magnoliaceae, which commonly grow in the valleys and mountains of China, Japan, and Korea. The two main bioactive compounds isolated from these plants are MG (5,5ʹ-diallyl-2,2ʹ-dihydroxybiphenyl) and Honokiol (3,5ʹ-diallyl-4,2ʹ-dihydroxybiphenyl) which are phenolic regioisomers. Thus, MG has emerged as a promising anti-cancer drug; nevertheless, its low bioavailability and solubility limit its potential clinical application. Therefore, developing a delivery system that can solve these limitations is essential. Magnolol also possesses a variety of pharmacological effects, including anti-oxidant, anti-inflammatory, anti-bacterial, anti-thrombotic or anti-platelet, anti-stress, anti-anxiety, anti-Alzheimer, Alzheimer, anti-stroke, hypoglycemic, smooth muscle relaxant, weight control, anti-dyspeptic/prokinetic, anti-epileptic and hepatoprotective effects.[2]
Magnolol is a natural polyphenolic compound that has demonstrated significant anti-cancer effects through multiple mechanisms, including inhibiting cell proliferation, the promotion of apoptosis, and the suppression of angiogenesis mediated by key signaling pathways. While MG’s low bioavailability and solubility have limited its clinical application, various formulation strategies, including nanoparticles and phospholipid complexes, have been developed to enhance its pharmacokinetics, showing promising results. Regarding safety and toxicity, MG has been tested in vitro and in vivo on different cancer cells and has been used in human clinical trials with no adverse side effects. Despite these advances, a complete understanding of its molecular mechanisms is still lacking, and further research is needed to elucidate its anti-cancer actions fully. This is crucial because despite its potent anti-cancer properties. Additionally, although Magnolol has shown low toxicity and is considered safe at lower doses, there is still a need for more comprehensive clinical trials to confirm its efficacy and safety in human populations. Therefore, while Magnolol holds considerable potential as an anti-cancer agent, further studies are essential to overcome existing challenges, such as improving its bioavailability, detailing the mechanisms in cancer cells that support evidence for antiproliferative activity, and expanding clinical data.
Safety and Toxicology of Magnolol
Neolignans, particularly magnolol and honokiol, are reported to be the compounds mainly responsible for MBEʼs beneficial properties, and their biological and pharmacological activities have been extensively investigated. The aim of this review is to have a critical look to the safety and toxicological properties of magnolol and honokiol, as pure substances or as components of concentrated MBE containing more than 90% of total neolignans, on the basis of a critical, systematic analysis of the literature. To retrieve all the pertinent toxicology evidence, we applied the PICOS conceptual framework and the applicable features of the PRISMA statement to a structured literature search using a multidatabase platform. The search was done on May 25, 2017, with a global geographical coverage and time limits from the year 1910 to the date of the search.[3]
Magnolia officinalis and Magnolia obovata bark extracts have been used for thousands of years in Chinese and Japanese traditional medicines and are still widely employed as herbal preparations for their sedative, antioxidant, anti-inflammatory, antibiotic, and antispastic effects. Neolignans, particularly magnolol and honokiol, are the main substances responsible for the beneficial properties of the magnolia bark extract (MBE). Here we review the safety and toxicological properties of magnolol and honokiol as pure substances or as components of concentrated MBE, including the potential side-effects in humans after oral intake. In vitro and in vivo genotoxicity studies indicated that concentrated MBE has no mutagenic and genotoxic potential, while a subchronic study performed according to OECD (Organisation for Economic Co-operation and Development) guidelines established a no adverse effect level for concentrated MBE > 240 mg/kg b.w/d. Similar to other dietary polyphenols, magnolol and honokiol are subject to glucuronidation, and despite a relatively quick clearance, an interaction with pharmaceutical active principles or other herbal constituents cannot be excluded.
References
[1]Zhang J, Chen Z, Huang X, Shi W, Zhang R, Chen M, Huang H, Wu L. Insights on the Multifunctional Activities of Magnolol. Biomed Res Int. 2019 May 23;2019:1847130. doi: 10.1155/2019/1847130. PMID: 31240205; PMCID: PMC6556366.
[2]Rayamajhi A, Gyawali N, Karki D, Pérez-Caltzontzin LE, Peña-Corona SI, Cortés H, Adhikari A, Leyva-Gómez G, Uprety Y, Habtemariam S, Kiyekbayeva L, Sharifi-Rad J. Magnolol and its semi-synthetic derivatives: a comprehensive review of anti-cancer mechanisms, pharmacokinetics, and future therapeutic potential. Discov Oncol. 2025 May 7;16(1):683. doi: 10.1007/s12672-025-02409-2. PMID: 40335865; PMCID: PMC12058641.
[3]Sarrica A, Kirika N, Romeo M, Salmona M, Diomede L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018 Nov;84(16):1151-1164. doi: 10.1055/a-0642-1966. Epub 2018 Jun 20. PMID: 29925102.
See also
Lastest Price from Magnolol manufacturers
US $0.00-0.00/kg2025-09-08
- CAS:
- 528-43-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1

US $5.00-0.50/KG2025-05-08
- CAS:
- 528-43-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS