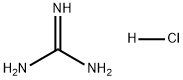
Why does guanidine hydrochloride denature proteins?
Sep 27,2025
The mechanism by which guanidine hydrochloride denatures proteins primarily involves the following aspects:Disruption of hydrogen bonds: Guanidine hydrochloride disrupts hydrogen bonds within protein structures, increases the solubility of non-polar residues, and reduces hydrophobic interactions, thereby inducing protein denaturation.
Electrostatic attraction: As a positively charged molecule, guanidine hydrochloride interacts electrostatically with negatively charged groups on proteins, leading to protein dissociation and disruption of their three-dimensional structure.
Complex formation: Denatured proteins preferentially bind to guanidine hydrochloride, forming denatured protein–denaturant complexes. As the concentration of the denaturant increases, native proteins are progressively converted into these complexes, ultimately resulting in complete denaturation.
Related articles And Qustion
Lastest Price from Guanidine hydrochloride manufacturers
Guanidine hydrochloride

US $10.00/kg2025-06-26
- CAS:
- 50-01-1
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100
Guanidine hydrochloride

US $1.00/KG2025-04-23
- CAS:
- 50-01-1
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 5tons/month