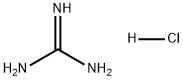
The Uses of guanidine hydrochloride
Background
Guanidine hydrochloride is an organic substance with the chemical formula CH6ClN3, white or yellowish lumps. It is used as an intermediate for medicines, pesticides, dyes and other organic compounds. It is an important raw material for the manufacture of sulfonamides and folic acid. It can also be used as an antistatic agent for synthetic fibers. At 20°C, 228 g can be dissolved in 100 g of water, 76 g can be dissolved in 100 g of methanol, and 24 g can be dissolved in 100 g of ethanol. Almost insoluble in acetone, benzene and ether. It can be used as intermediates in medicine, pesticides, dyes and other organic synthesis. It can be used to synthesize 2-aminopyrimidine, 2-amino-6-methylpyrimidine, and 2-amino-4,6-dimethylpyrimidine. drug intermediates. Guanidine hydrochloride (or guanidine nitrate) reacts with ethyl cyanoacetate to form 2,4-diamino-6-hydroxypyrimidine, which is used to synthesize the anti-anemia drug folic acid. It can also be used as an antistatic agent for synthetic fibers. Used as an organic synthesis intermediate; used as an antistatic agent for synthetic fibers; a strong chaotropic agent for protein denaturation and subsequent renaturation[1].

Picture 1 Guanidine hydrochloride powders
Properties
This product is relatively unstable and can be hydrolyzed in aqueous solution to generate ammonia and urea, so its toxicity is the same as urea, and guanidine and its derivatives are generally more toxic than urea. [2]
Application of guanidine hydrochloride
Guanidine hydrochloride can be used as pharmaceuticals, pesticides, dyes and other organic synthesis intermediates. It can be used to synthesize 2-aminopyrimidine, 2-amino-6-methylpyrimidine, 2-amino-4,6-dimethylpyrimidine, and is used for the manufacture of sulfadiazine, sulfamethazine, sulfamethazine and other sulfa drugs. Intermediate. Guanidine hydrochloride (or guanidine nitrate) reacts with ethyl cyanoacetate to form 2,4-diamino-6-hydroxypyrimidine, which is used to synthesize the anti-anemia drug folic acid. It can also be used as an antistatic agent for synthetic fibers. Can also be used for protein denaturants. As a strong denaturant in experiments for extracting total cellular RNA[3]. The guanidine hydrochloride solution can dissolve the protein, leading to the destruction of the cell structure, the destruction of the secondary structure of the nucleoprotein, and the dissociation from the nucleic acid. In addition, the RNase can be inactivated by reducing agents such as guanidine hydrochloride.
Preparation
Using dicyandiamide and ammonium salt (ammonium chloride) as raw materials, the melting reaction is carried out at 170-230 ° C to obtain crude guanidine hydrochloride, which is refined to obtain the finished product.
The role of urea and guanidine hydrochloride in protein purification of inclusion bodies
Denaturing agents (urea and guanidine hydrochloride) can break hydrogen bonds in the protein structure, which increases the solubility of non-polar molecules, including amino acid side chains, and reduces hydrophobic interactions; urea can also penetrate deep into protein molecules and affect proteins close packing of molecules. In addition, detergents, organic solvents, heavy metals, heat, mechanical force, freezing, ultrasound, high pressure, radiation, etc. can cause protein denaturation. These denaturations do not destroy the covalent bonds of proteins but only involve the destruction of secondary bonds such as hydrogen bonds, salt bonds, hydrophobic interactions, and Van der Waals interactions. Some denatured proteins can automatically return to their natural state after the denaturation factor is removed. This phenomenon is called protein renaturation. This renaturation is the refolding in the study of protein folding. Most inclusion body proteins are purified under the condition of pH 8. Generally, strong denaturing agents such as urea (6-8 mol/L), guanidine hydrochloride (GdnHCl 5-8 mol/L or 6 mol/L) are used. In general, guanidine hydrochloride is better than urea, because guanidine hydrochloride is a stronger denaturant than urea, and it can dissolve inclusion bodies that cannot be dissolved by urea.
Furthermore, urea-decomposed isocyanates can lead to free carbamylation of polypeptide chains, especially when incubated at alkaline pH for long periods of time. Or use detergents, such as SDS, n-hexadecyltrimethylammonium chloride, Sarkosyl, etc., can destroy the hydrophobic bonds in the protein, and can also dissolve some inclusion body proteins. Zymononasmobilislevansucrase inclusion body protein was solubilized with TritonX-100. In addition, for cysteine-containing proteins, the isolated inclusion bodies usually contain some interchain formed disulfide bonds and some intrachain inactive disulfide bonds.
There are also organic solvents, alkaline environments (greater than 9) or acids (70% formic acid) that can also dissolve it. A reducing agent should be added to the denaturing solution, such as 2-mercaptoethanol (β-ME), dithiothreitol (DTT), dithioerythritol, reduced glutathione (GST), cysteine acid. The concentration of reducing agent used is generally 50-100 mM β-ME or DTT, and there are also literatures using 5 mM concentration. The concentration of reducing agent used has nothing to do with the number of protein disulfide bonds Chemicalbook, and some proteins without disulfide bonds with or without reducing agent have no effect, such as the solubilization of bovine growth hormone inclusion bodies. For the solubilization of some inclusion bodies without disulfide bonds in the target protein, sometimes the use of reducing agents is also necessary, which may be due to the influence of the solubilization of inclusion bodies by heteroproteins containing disulfide bonds. At the same time, it is necessary to add a metal chelating agent such as EDTA or EGTA to chelate metal ions such as Cu2+ and Fe2+, and prevent the sulfhydryl group in the reduced state from oxidizing with it. After the denaturant dissolves, the protein loses its biological activity.
Gumecycline Hydrochloride Capsules
This product is capsule, the content is yellow powder. For respiratory infections caused by susceptible bacteria. oral. 1.2g (12 capsules) a day, divided into 3~4 times. Sealed and stored in a dry cool dark place.
This product is a broad-spectrum bacteriostatic agent, which has bactericidal effect at high concentration. This product is effective against Streptococcus pneumoniae, Streptococcus hemolyticus, Escherichia coli and some Staphylococcus, Clostridium perfringens, Bacillus anthracis, Yersinia pestis, Diphtheria, Tetanus, Brucella, Haemophilus influenzae, flexure Bacillus and Vibrio cholerae have certain antibacterial effects, and also have inhibitory effects on rickettsia, mycoplasma, chlamydia, spirochetes and some protozoa. Enterobacteriaceae bacteria such as Escherichia coli, Klebsiella, Salmonella, Shigella, etc. are mostly resistant to this product. The effect of this product on Gram-positive bacteria is better than that of Gram-positive bacteria, but Enterococcus is resistant to it. Others such as Actinomyces, Bacillus anthracis, Listeria monocytogenes, Clostridium, Nocardia, etc. are also sensitive to this product. This product is also resistant to tetracycline against gonococcus. This product has certain antibacterial activity against Neisseria gonorrhoeae and Neisseria meningitidis, but penicillin-resistant gonococcus is also resistant to tetracycline. The mechanism of action of this product is that the drug can specifically bind to the A position of the 30S subunit of the bacterial ribosome, inhibiting the connection of aminoacyl-tRNA at this position, thereby inhibiting the growth of the peptide chain and affecting the synthesis of bacterial proteins.
This product has great solubility in water, and is rapidly absorbed after oral administration. The blood concentration is the highest in 3 hours, and the effective blood concentration can be maintained for 12 hours. Compared with other tetracyclines, its main feature is that it has a greater affinity for bronchial and lung tissue, and the concentration in bronchial, lung tissue and bronchial secretions after taking the drug is significantly higher than that of tetracycline hydrochloride taking a comparable dose. The blood concentration is higher and lasting than that of tetracycline. Mainly excreted in the urine in the active form.
Reference
1 Chen Jing, Li Haozhou, Zhang Yun, Sun Shiqi, Fang Shouguo, Guo Huichen, Yang Yuying. Effects of guanidine hydrochloride on intact particles and empty capsids of foot-and-mouth disease virus [J]. Journal of Virology, 2022,38(02):415-421.
2 Xia Xueting, Wang Yuqiang, Yang Jianjun, Wu Qingyun, Wu Mingyuan, Zhang Jianan, Liu Jiuyi. Preparation and Properties of Molybdenum Disulfide Supported Polyhexamethyleneguanidine Hydrochloride Antibacterial Waterborne Polyurethane [J]. Fine Chemical Industry, 2022,39(02): 288-294.
3 Cheng Peng, Guo Zongwei, Xu Yanglei, Li Xin, Song Yijia, Xu Feng. Mild and efficient separation of bamboo lignin with formic acid/guanidine hydrochloride deep eutectic solvent[J]. Fine Chemical Industry, 2022,39(02):345-351.
Related articles And Qustion
Lastest Price from Guanidine hydrochloride manufacturers

US $10.00/kg2025-06-26
- CAS:
- 50-01-1
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100

US $1.00/KG2025-04-23
- CAS:
- 50-01-1
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 5tons/month