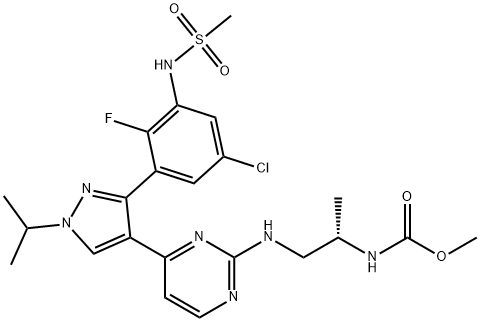
A BRAF inhibitor: Encorafenib (LGX818)
Description
Encorafenib (LGX818) is a BRAF inhibitor, and detection of a BRAF V600E or BRAF V600K mutation in tumor specimens is required before treatment. Approximately 50% of melanomas contain an activating BRAF V600 mutation[1].
Encorafenib and binimetinib
Encorafenib was approved by the USFDA in 2018 as a combination treatment with binimetinib for unresectable or metastatic melanoma. These combination therapies have become the standard of patient care in such cases. While initial response rates are often highly promising, treatment resistance and progression occur in approximately 80% of cases within 3 years. In clinical trials, patients given the combination of encorafenib and binimetinib had an increased progression-free survival (PFS) and overall survival (OS) compared to encorafenib or vemurafenib, a USFDA-approved treatment for late-stage melanoma. Encorafenib is currently marketed in the U.S. by Pfizer after it acquired Array BioPharma in 2019.
Pharmacokinetics
As an ATP-competitive v-Raf murine sarcoma viral oncogene homolog (RAF) serine and threonine kinase inhibitor, Encorafenib selectively exhibits antiproliferative effects in BRAF V600E-mutated cells[2]. In humans, oral encorafenib is highly bioavailable (around 85%) and rapidly absorbed with a median time to the maximum observed concentration (Tmax) of approximately 2-h postdose. The plasma elimination half-life of encorafenib is around 6 h. Elimination of encorafenib occurs mainly through metabolism via cytochrome P450 (CYP) enzymes (CYP3A4, CYP2C19 and CYP2D6).
Synthetic method
Synthesis of Encorafenib Carbamate 259
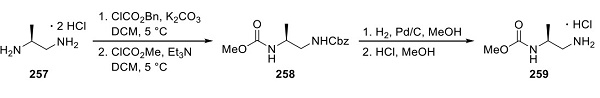
Novartis disclosed a synthesis of encorafenib. The convergent route hinged upon constructing the central pyrazole core, onto which substituted aryl and heteroaryl groups were appended. (S)-(−)-1,2-Diaminopropane dihydrochloride (257, shown above) was treated with potassium carbonate to form the free base, followed by sequential addition of benzyl chloroformate and methyl chloroformate to give bis-carbamate 258. Hydrogenation with palladium on carbon then provided the chiral carbamate fragment 259.
Synthesis of Encorafenib Boronate Ester 263
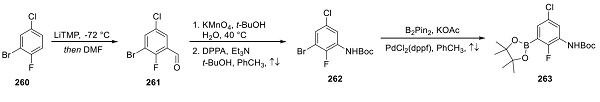
Preparation of encorafenib also necessitated the construction of an aryl boronate ester, and this approach began with the formulation of 2-bromo-4-chloro-1-fluorobenzene (260) to give benzaldehyde 261 (shown above). Oxidation to the carboxylic acid followed by Curtius rearrangement delivered Boc-aniline 262, which was then converted to boronic ester 263 under Miyaura conditions involving bis(pinacolato)- diboron.
Synthesis of Encorafenib
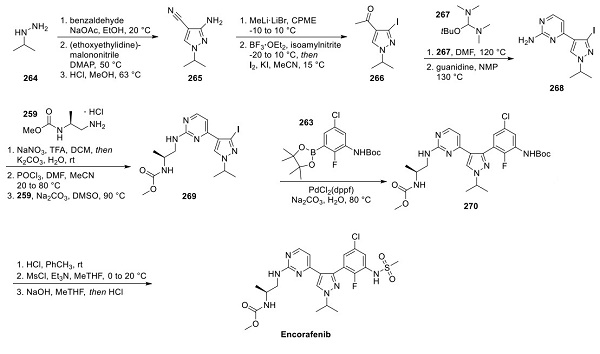
Synthesis of the pyrazole core began with benzyl protection of isopropylhydrazine 264 (shown above), followed by alkylation with (ethoxyethylidine)malononitrile. Acid-promoted cyclization furnished aminopyrazole 265. Methyl ketone formation made way for a Sandmeyer conversion of the pendant amine to the corresponding iodide, providing iodopyrazole 266. The methyl ketone was then converted to 2- aminopyrimidine 268 in two steps: first, by the addition of Brederick’s reagent (267), and second, by cyclization with guanidine. Formal hydrolysis to the pyrimidone was accomplished with a second Sandmeyer reaction, and subjection to phosphorus oxychloride and carbamate 259 ultimately delivered ethylenediamine 269. After Suzuki cross-coupling with boronic ester 263, intermediate 270 was acidified to reveal the aniline, which was then treated with methanesulfonyl chloride to generate the bis-mesylated form of the drug target. Careful exposure to sodium hydroxide and subsequent acidification resulted in selective monosulfonyl deprotection to furnish encorafenib.
References
[1] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
[2] Peter Koelblinger, Reinhard Dummer, Olaf Thuerigen. “Development of encorafenib for BRAF-mutated advanced melanoma.” Current Opinion in Oncology 30 2 (2018): 125–133.
You may like
See also

US $0.00/g2025-01-13
- CAS:
- 1269440-17-6
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $15.00-10.00/KG2021-07-13
- CAS:
- 1269440-17-6
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton