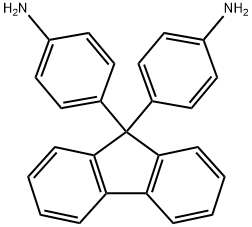
9,9-Bis(4-aminophenyl)fluorene: Applications in Solar Cells, Polyimides, and Polymer Synthesis
9,9-Bis(4-aminophenyl)fluorene (FDA) is a fluorene derivative with two aminophenyl substituents on the 9-position. This compound has partial conjugation and can be utilized as a dopant-free organic hole-transporting material in inverted perovskite solar cells. Due to its diaminophenyl groups, FDA is commonly employed in synthesizing polyimides that possess high thermal and hydrolytic stability for optical fiber light guide coatings. Because the FDA is not fully aromatized, it is chosen to produce thermally stable transparent polyimides. Additionally, the polyimide derived from 9,9-bis(4-aminophenyl)fluorene can be further modified through Friedel-Crafts alkylation on the fluorene moiety, as the polyimide backbone remains chemically stable.

Poly(pyridinium salt)s Containing 9,9-Bis(4-aminophenyl)fluorene Moieties
Phenylated poly(pyridinium salt)s are a class of main-chain cationic polymers known as ionenes. They are usually prepared by the ring-transmutation polymerization reaction of bispyrylium salts and diamines and metathesis reactions. Depending on the chemical structures of bispyrylium salts and diamines, they can be π-conjugated or non-conjugated ionenes. Various non-conjugated ionenes exhibit thermotropic liquid-crystalline (LC) and light-emitting properties in both solution and solid state. Additionally, π-conjugated and even non-conjugated ionic polymers exhibit lyotropic LC phases in both protic and aprotic solvents and light-emitting properties in both solution and solid state depending on their chemical microstructures. The 4,4′-(1,4-phenylene)bis(2,6-diphenylpyrylium)ditosylate, M, was synthesized according to the reported procedure. The 9,9-bis(4-aminophenyl)fluorene was purchased from Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan, and purified by recrystallization from chloroform/hexane. It showed a melting endotherm (Tm) at peak maximum of 237 °C in the DSC thermogram obtained at a heating rate of 10 °C/min. Main-chain conjugated and non-conjugated polyelectrolytes are an important class of materials that have many technological applications ranging from fire-retardant materials to carbon-nanotube composites, nonlinear optical materials, electrochromic materials for smart windows, and optical sensors for biomolecules. Here, we describe a series of poly(pyridinium salt)s-fluorene containing 9,9-bis(4-aminophenyl)fluorene moieties with various organic counterions that were synthesized using ring-transmutation polymerization and metathesis reactions, which are non-conjugated polyelectrolytes.[1]
A comparative study of 9,9-bis(4-aminophenyl)fluorene polymers
Oxidative polymerisation (OP) is one of the important green chemistry processes to prepare multifunctional polymers. This method has been widely used to prepare different types of polymers such as polyaniline, polypyrrole, polythiophene, polyphenol, polyfluorene, polycarbazole, polySchiffbases and their different derivatives. 9,9-Bis(4-aminophenyl)fluorene is a commercially available compound possessing both aromatic amine groups and fluorene rings in its structure. This compound is very susceptible to OP via oxidation of fluorene and amino groups. We assume that APF can combine the advantages of aromatic amine and fluorene moieties. From this point of view, it can be foreseen that kind of linkage between monomer units is of crucial importance to determine the physical properties of new polymers. In typical polymerisation, 9,9-Bis(4-aminophenyl)fluorene (0.01 mol) was dissolved in an aqueous solution of KOH (10%, 0.001 mol) and placed into a 50 ml three-necked round-bottom flask. It was fitted with a condenser, thermometer, stirrer and an addition funnel containing NaOCl. After heating to 70 °C, NaOCl was added drop by drop over about 20 min. Reaction was maintained for 5 h. The mixture was neutralized with 0.001 mol HCl (37%) at room temperature. Unreacted monomer was separated from the reaction products by washing with acetonitrile.[2]
9,9-Bis(4-aminophenyl)fluorene was oxidatively polymerised in both aqueous alkaline and organic medium. The spectral, thermal, optical, electrical, electrochemical, and morphological characteristics of the products obtained with different reaction media are completely different from each other. NMR analysis showed that the polymerisation of 9,9-Bis(4-aminophenyl)fluorene in alkaline medium selectively proceeded to form the polymer linked by o-positions of phenyl rings. It can be concluded that the choice of the solvent as well as the type of linkage in polymer chains has a significant role in deciding the properties of resulting polymer like conductivity and morphology. It was observed that the obtained polyfluorene product via catalytic oxidative polymerisation has higher fluorescence properties than that obtained by the other method. This means the polymerisation on the fluorene units mainly responsible for the higher fluorescence efficiency.
References
[1]Bhowmik PK, King D, Han H, Wacha AF, Knaapila M. Poly(pyridinium salt)s Containing 9,9-Bis(4-aminophenyl)fluorene Moieties with Various Organic Counterions Exhibiting Both Lyotropic Liquid-Crystalline and Light-Emitting Properties. Polymers (Basel). 2025 Jun 27;17(13):1785. doi: 10.3390/polym17131785. PMID: 40647794; PMCID: PMC12252291.
[2]Bilicı, A., Kaya, İ., & Yıldırım, M. (2011). A comparative study of 9,9-bis(4-aminophenyl)fluorene polymers prepared by catalytic and non-catalytic oxidative polymerisation methods. European Polymer Journal, 47(5), 1005-1017. https://doi.org/10.1016/j.eurpolymj.2011.02.019
You may like
Lastest Price from 9,9-Bis(4-aminophenyl)fluorene manufacturers

US $0.00-0.00/kg2025-10-27
- CAS:
- 15499-84-0
- Min. Order:
- 1kg
- Purity:
- ≥99.5%
- Supply Ability:
- 30TM
US $0.00/kg2025-09-11
- CAS:
- 15499-84-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons