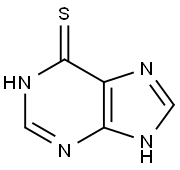
6-Mercaptopurine: Mechanism of Action and Mechanism of Resistance
Mechanism of Action
6-Mercaptopurine has been used as an antineoplastic and immunosuppressive agent for decades, but the precise mechanism by which it exerts its cytotoxic effects has not yet been established. Both 6-MP and 6-TG must be converted to their thiol nucleotide form, which is the active cytotoxic moiety. The conversion is catalyzed by hypoxanthine guanine phosphoribosyltransferase (HGPRT) and the reaction is dependent on the phosphoribosylpyrophosphate (PRPP) level in the cells. 6-Thiouric acid (6-TU) is the major catabolic product of 6-MP. The rapid conversion of 6-MP to 6-TU by xanthine oxidase, in leukemic cells may be a possible mechanism of 6-MP resistance. The antineoplastic effect of 6-TG is similar to that of 6-MP. In its nucleotide form, 6-TG inhibits de novo purine biosynthesis and purine interconversions.

The cytotoxicity of 6-MP and 6-TG has been
linked to (1) the interference with de novo purine
biosynthesis and purine interconversions, (2) the
inhibition of in vitro RNA synthesis, and (3) the
incorporation into DNA during S phase, resulting in a deformation of the DNA.
The interference with de novo purine biosynthesis by 6-MP is regulated by 6- MP nucleotides. These nucleotides inhibit the enzyme 5-phosphoribosylpyrophosphate amidotransferase that catalyzes the initial reaction in the purine biosynthetic pathway. The 6-MP nucleotides also inhibit conversion of inosine monophosphate (IMP) to adenine monophosphate (AMP) and to xanthine monophosphate (XMP), and limit the availability of XMP to form guanine monophosphate (GMP), thereby interfering with the supply of purine precursors for nucleic acid synthesis. In the 1980s two new findings pertaining to the cytotoxicity mechanism in tumor cells have been reported. Studies of human lymphoma revealed that 6-MP was a potent inhibitor of cellular RNA synthesis and that 6-thioITP inhibited both the RNA polymerase I and RNA polymerase II activities of these cells. These data suggested that direct inhibition of the enzymes mediating transcription by 6-thio-IMP may be one of the mechanisms for the cytotoxic action of 6-MP in human tumor cells. Using 6- TG as a cytotoxic agent resulted in severe chromosome damage in wild-type CHO cells. Gross unilateral chromatid damage resulted, and the unilateral nature of this damage was probably due to malfunction of 6-TG-containing DNA as a replication template.
Mechanism of Resistance
Several mechanisms of resistance to these agents have been described in experimental tumors and relate to the pathways of antimetabolite activation and degradation. A decreased HGPRT activity in tumor cells diminishes antimetabolite activation, and this resistance pathway has been reported by several workers. However, the HGPRT-regulated mechanism as a basis for drug resistance in human leukemic cells is relatively uncommon. The resistance of 6-MP is related to an increase in alkaline phosphatase in sarcoma cells. Increased alkaline phosphatase, a membrane-bound enzyme that converts the active mononucleotide to 6- thioinosine and inorganic phosphate, has also been reported in human leukemia patients resistant to drug treatment. Another enzyme in the degradation pathway of 6-MP that needs to be considered for drug efficacy and bioavailability is xanthine oxidase, which is responsible for converting 6-MP to 8-OH-6-MP and subsequently to thiouric acid, which is excreted through the urine.
To understand further the bioavailability and pharmacokinetics of thiopurines, the effect of allopurinol on 6-MP catabolism was studied. Allopurinol, an analog of hypoxanthine, enhances the therapeutic efficacy of 6-MP by inhibiting xanthine oxidase. The urinary excretion of 6- MP metabolites is markedly reduced in patients treated with allopurinol. Furthermore, allopurinol increased the plasma level of 6-MP in rabbits. The data suggest that inhibition of 6- MP catabolism by allopurinol may contribute to a greater availability of 6-MP to tissues. Studies of the effect of allopurinol on the kinetics of oral and intravenous 6-MP in Rhesus monkeys and in humans demonstrated that allopurinol pretreatment resulted in a nearly 400 % increase in peak plasma concentration of 6-MP in monkeys and a 500 % increase in humans, but only when 6-MP was administered orally. Allopurinol pretreatment had no effect on the kinetics of intravenously administered 6-MP. This difference is due to the action of allopurinol on liver or intestinal xanthine oxidase and inhibition of first-pass metabolism of oral 6-MP. This finding may explain the low and variable plasma levels of mercaptopurine in patients with acute lymphoblastic leukemia treated with oral 6-MP. Although these studies emphasize the catabolic pathway for purines, all the purine and pyrimidine metabolic enzymes may be important to the bioavailability and activation of antimetabolites.
To clarify the mechanisms of resistance to thiopurines and potential drug interactions in tumor cells, studies have focused on the thiopurine-resistant cell lines deficient in HGPRT (L 1210) and the regulation of PRPP formation in thiopurine-resistant cell lines.
The thiopurines are inactive in the base form and must be converted to their respective nucleotides. This activation step requires PRPP as the cofactor and HGPRT as the enzyme to catalyze the conversion to nucleotide. A major biochemical effect of methotrexate is the suppression of purine biosynthesis and expansion of the PRPP pool. Studies of the cytotoxic and biochemical interaction of methotrexate and 6-TG in L 1210 mouse leukemia cells demonstrated that methotrexate can markedly enhance 6-TG activity. Preexposure of cells to methotrexate resulted in a large increase in cytotoxic potency of 6-TG, whereas simultaneous exposure caused an antagonism of 6-TG cytotoxic activity. Although PRPP pools were not measured quantitatively, the effect of methotrexate preexposure was to increase PRPP pools, enhance the activation of 6-TG to 6-TG monophosphate, and thereby increase its incorporation into RNA.
You may like
Lastest Price from 6-Mercaptopurine manufacturers

US $1.00/KG2025-03-14
- CAS:
- 50-44-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00/kg2025-03-03
- CAS:
- 50-44-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS