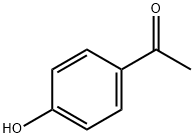
4'-Hydroxyacetophenone: Pharmacological Activity and Catabolic Pathway
General Description
4'-Hydroxyacetophenone, a compound found in plants like Artemisia, possesses antioxidant, anti-inflammatory, and antibacterial properties. 4'-Hydroxyacetophenone is used in cosmetics and pharmaceuticals, and medicinally for treating hepatitis and jaundice. Pharmacologically, 4'-Hydroxyacetophenone activates nonmuscle myosin-2B and -2C, showing promise in reducing metastatic burden in colorectal and pancreatic cancer models. Understanding its catabolic pathway in Pseudomonas fluorescens ACB reveals a multi-step degradation process involving specific enzymes. This research sheds light on 4'-Hydroxyacetophenone's potential therapeutic applications and highlights its significance in both medical and microbial contexts.

Figure 1. 4'-Hydroxyacetophenone
Overview
4'-Hydroxyacetophenone, also known as p-Hydroxyacetophenone or 4-Hydroxyacetophenone, is a white needle-like crystal with a mild sweet hawthorn balsam aroma. 4'-Hydroxyacetophenone is soluble in hot water, methanol, ethanol, and other solvents but insoluble in petroleum ether. This compound naturally occurs in plants like Artemisia and Radix Adenophorae. 4'-Hydroxyacetophenone exhibits antioxidant, anti-inflammatory, and antibacterial properties, making it a valuable ingredient in cosmetics and pharmaceuticals. In medicine, 4'-Hydroxyacetophenone is used to treat hepatitis and jaundice due to its ability to increase bile secretion. However, ingestion of this compound can be harmful, and it should be handled with caution. 1
Pharmacological Activity
4'-hydroxyacetophenone exhibits pharmacological activity by activating nonmuscle myosin-2B (NM2B, Myh10) and -2C (NM2C, Myh14) through the promotion of their assemblies. Research indicates that 4'-hydroxyacetophenone plays a significant role in reducing metastatic burden in an in vivo model of colorectal cancer liver metastases. The treatment with 4-HAP induces actin remodeling via an NM2C-dependent mechanism, consequently inhibiting the cells' ability to polarize, disseminate, and invade. Studies have also shown that 4'-hydroxyacetophenone exerts similar effects on metastasis in pancreatic cancer models, particularly targeting NM2C. Given that pancreatic cancer cells express NM2C exclusively, while colon cancer cells express both NM2B and NM2C, it is evident that NM2C is the primary focus of 4'-hydroxyacetophenone's antimetastatic action in colon cancer. This research underscores the potential of modulating the actomyosin cytoskeleton as a promising avenue for future cancer therapies aimed at suppressing metastatic progression. By targeting and activating NM2C, the molecular machinery responsible for regulating cell shape and function in various processes including cancer metastasis, there is an opportunity to enhance current therapeutic strategies. The findings highlight the importance of exploring novel approaches to combat metastatic diseases and offer insights into a strategy that influences cytoskeletal plasticity, demonstrating efficacy both in laboratory settings and animal models. This approach could be integrated synergistically with existing treatments for aggressive cancers. 2
Catabolic Pathway
The catabolic pathway of 4'-hydroxyacetophenone in Pseudomonas fluorescens ACB involves several key steps, elucidated through genetic and biochemical analyses. Initially, 4'-hydroxyacetophenone is metabolized to hydroquinone. Further degradation of hydroquinone occurs via intermediate steps to eventually yield β-ketoadipate. This process involves several enzymes encoded by specific genes within the P. fluorescens ACB genome. A 15-kb DNA fragment analysis revealed a gene cluster (hapCDEFGHIBA) containing 14 open reading frames, with at least four enzymes (hapA, hapB, hapE, and hapF) directly involved in 4'-hydroxyacetophenone degradation. Specifically, HapA functions as a 4'-hydroxyacetophenone monooxygenase, while HapB acts as a 4'-hydroxyphenyl acetate hydrolase. Additionally, HapE serves as a 4'-hydroxymuconic semialdehyde dehydrogenase, and HapF functions as a maleylacetate reductase. Within this gene cluster, other genes (hapG, hapH, and hapI) encode proteins involved in subsequent steps of the pathway, including a putative intradiol dioxygenase, a protein of the Yci1 family, and a [2Fe-2S] ferredoxin, respectively. Downstream of the hap genes, regulatory proteins and proteins potentially involved in a membrane efflux pump are also present. Notably, two genes upstream of hapE (hapC and hapD) are implicated in the ring cleavage of hydroquinone, suggesting their involvement in an iron(II)-dependent extradiol dioxygenase mechanism. Overall, the elucidation of the catabolic pathway of 4'-hydroxyacetophenone in Pseudomonas fluorescens ACB provides valuable insights into the microbial degradation of aromatic compounds and highlights the complexity of bacterial metabolic pathways. 3
Reference
1. 4'-Hydroxyacetophenone. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 7469.
2. Bryan DS, Stack M, Krysztofiak K, et al. 4-Hydroxyacetophenone modulates the actomyosin cytoskeleton to reduce metastasis. Proc Natl Acad Sci U S A. 2020;117(36):22423-22429.
3. Moonen MJ, Kamerbeek NM, Westphal AH, et al. Elucidation of the 4-hydroxyacetophenone catabolic pathway in Pseudomonas fluorescens ACB. J Bacteriol. 2008; 190(15): 5190-5198.
You may like
Related articles And Qustion
Lastest Price from 4'-Hydroxyacetophenone manufacturers

US $30.00-20.00/kg2025-11-11
- CAS:
- 99-93-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5mt
US $70.00/kg2025-08-29
- CAS:
- 99-93-4
- Min. Order:
- 800kg
- Purity:
- 99%
- Supply Ability:
- 50 ton per month