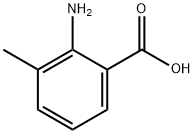
3-Methylanthranilic acid: Biological Effects, and Protonation Equilibria Studies
3-Methylanthranilic acid is an aromatic amino acid that is anthranilic acid in which one of the hydrogens attached to the nitrogen is substituted by a methyl group. It has a role as a plant metabolite and is a pink to grey-brown crystalline powder with potential applications in various industries due to its unique chemical properties.

Synthesis of 3-Methylanthranilic Acid
A 150 ml pressure reactor with overhead stirring was charged with water, Raney -Nickel catalyst, and 1.05 equivalents of 50% aqueous sodium hydroxide. About 0.065 grams of Raney -Nickel slurry (50% water) was charged, so the mass equivalence to starting material was about 3.24 wt%. The 3-Methylanthranilic acid was charged and produced a thin greenish-colored solution. The reactor was sealed and pressure-purged with N2 three times to remove air. The reactor was pressure-tested using N2 and then the reactor was pressure-purged with hydrogen gas three times. The reactor was pressurized with to starting pressure (300 psi) and hydrogen line was kept open, so the system was continuously supplied with as it was used up during reaction. The reactor agitation was set at 800 RPM and was heated to 80-100°C. Hydrogen gas was fed into the reactor for one hour. After reaction was considered complete, the reactor was cooled, the pressure was vented, and a sample of 3-Methylanthranilic acid was taken. HPLC analysis determined that the starting material was almost completely converted.[1]
3-Methylanthranilic Acid Derepression of the Tryptophan Operon
3-Methylanthranilic acid (3MA) inhibits growth and causes derepression of the tryptophan biosynthetic enzymes in wild-type strains of Escherichia coli. Previous reports attributed this effect to an inhibition of the conversion of 1-(o-carboxyphenylamino)-1-deoxyribulose 5-phosphate to indole-3-glycerol phosphate and a consequent reduction in the concentration of endogenous tryptophan. Our studies have shown that 3-Methylanthranilic acid -resistant mutants linked to the tryptophan operon have a feedback-resistant anthranilate synthetase; mutants with an altered indole-3-glycerol phosphate synthetase were not found. 3MA or 7-methylindole can be metabolized to 7-methyltryptophan, and 3-Methylanthranilic acid, 7-methylindole, and 7-methyltryptophan lead to derepression of the tryptophan operon. Furthermore, 3MA-resistant mutants are also resistant to 7-methylindole derepression. These results strongly suggest that the primary cause of derepression by 3-Methylanthranilic acid is through its conversion to 7-methyltryptophan, which can inhibit anthranilate synthetase, thereby decreasing the concentration of endogenous tryptophan. Unlike 5- or 6-methyltryptophan, 7-methyltryptophan does not appear to function as an active corepressor.[2]
Microscopic Protonation Equilibria of Anthranilic Acid and Its Derivatives
This work is focused on three zwitterionic compounds, namely anthranilic (2-aminobenzoic acid), 3-methylanthranilic and 3-phenylanthranilic acids. These compounds play very important roles in biological and chemical processes. Anthranilic acid is the precursor of l-tryptophan in plants, yeast and bacteria and is also involved in the biosynthesis of phytohormones (auxins) and their precursors. it was shown that N-methylation of anthranilic acid to 3-methylanthranilic acid is the first pathway-specific reaction in acridone alkaloid biosynthesis. The pH-metric titration procedure has previously been described in detail. Titration of N-methylanthranilic acid solution was performed in analogy with the anthranilic acid solution but the N-methylanthranilic acid concentration was 0.002 mol·dm−3 and the concentration of the titrant (KOH solution) was 0.0196 mol·dm−3. For each sample three parallel measurements were performed and pK a values were calculated using the procedure described in Sect. 2.5. Titration data and the appropriate statistical parameters on anthranilic acid were presented in while these parameters for 3-methylanthranilic acid are summarized.[3]
The pK a (for anthranilic and 3-methylanthranilic acid) and ps K a (for N-phenylanthrnilic acid) can be deduced from the half-neutralization point of the titration curve but it is advantageous to determine the ionization constant from a difference curve (called formation curve or Bjerrum plot). It is the plot of ¯𝑛H (the average number of bound protons) against pH. The stock solutions of anthranilic and 3-methylanthranilic acid (0.040 mol·dm−3) were prepared in methanol and of N-phenylanthranilic (3.0 × 10−3 mol·dm−3) in MDM. 1 cm3 of each of these solutions was diluted to 200 cm3 with methanol or Britton–Robinson buffer (pH = 3.51, 3.94, 3.78 for anthranilic, 3-methylantranilic, 3-phenylanthranilic acids, respectively).
The absorption spectra of all studied compounds are characterized by two bands. A longer wavelength band for anthranilic acid has a maximum at 326 nm (in Britton–Robinson buffer) and in the case of 3-methylanthranilic and 3-phenylanthranilic acids, this is red shifted to about 341 and 346 nm. In turn, a shorter wavelength band for anthranilic and N-methylanthranilic acids have a maximum at about 240 and 247 nm, respectively that is not very distinct. On the other hand, in the case of 3-phenylanthranilic acid, this band is very distinct and the maximum is red shifted to about 283 nm. As described in the literature, the shorter wavelength band is ascribed to the π–π* transition of the benzenoid system, whereas the longer wavelength band can be attributed to the π–π* transition within the heterocyclic moiety (IHB ring) of the studied compounds. It was also observed that, with an increase of methanol content, the maximum in the shorter wavelength band is more distinct both for anthranilic and 3-methylanthranilic acids. Furthermore, the addition of methanol causes all absorption spectra to be red shifted relative to those in Britton–Robinson buffers. The influence of the type of substituent and pH of the aqueous phase on the equilibrium were analyzed with regard to the formation and the coexistence of different forms of the acids in the examined systems. Recapitulating, the potentiometry and spectrophotometric methods seem to be suitable for the study of intramolecular interactions of the aromatic amino acids containing both acidic and basic groups in the ortho position.
References
[1]FMC& - WO2022/232366, 2022, A1
[2]Held WA, Smith OH. Mechanism of 3-methylanthranilic acid derepression of the tryptophan operon in Escherichia coli. J Bacteriol. 1970 Jan;101(1):209-17.
[3]Zapa?a L, Wo?nicka E, Kalembkiewicz J. Tautomeric and Microscopic Protonation Equilibria of Anthranilic Acid and Its Derivatives. J Solution Chem. 2014;43(6):1167-1183.
You may like
See also
Lastest Price from 3-Methylanthranilic acid manufacturers

US $0.00/kg2025-08-22
- CAS:
- 4389-45-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-06-07
- CAS:
- 4389-45-1
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg