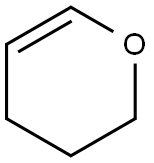
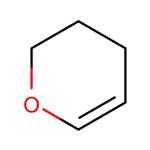
3,4-Dihydro-2H-pyran: a hydroxyl-protecting reagent
Widely-used hydroxyl-protecting reagent 3,4-Dihydro-2H-pyran is used as a hydroxyl-protecting reagent in organic synthesis. It acts as an intermediate in synthetic chemistry. It is used to protect various reactive functional groups. It is involved in the polymerization reaction either alone or with unsaturated compound.

Synthesis of 3,4-Dihydro-2H-pyran
To the resulting slurry was added 125 ml. of dihydropyran and then 13 drops of conc. HCl were added. The clear, straw-colored reaction solution was stirred at room temperature for approximately 3 hours, transferred to a separatory funnel, washed with about 400 ml. of aqueous 10% sodium hydroxide and the dichloromethane layer dried over anhydrous sodium sulfate. After drying, the dichloromethane solution was filtered through fresh anhydrous sodium sulfate, and the pale straw filtrate was evaporated under reduced pressure leaving 25.9 g. of straw yellow oil. The oil was applied directly to a wet packed SiO2 column (100-200 mesh: 4/1 petroleum ether/ether) and eluted with 4/1 petroleum ether/ether. Twenty-four fractions of about 50 ml. each were collected, and fractions 9-24 were combined and evaporated to give 16.91 g. of straw syrup which upon standing crystallized to give the title compound as pale lemon crystals. The 2'-tetrahydropyranyl ether of 4-bromo-2-chloro-1-naphthol also was prepared according to the foregoing procedure. The methoxymethylation of 4-bromo-1-naphthol was carried out as follows: To a stirred solution of 1.0 g. of 4-bromo-1-naphthol in 20 ml. of chloroform (previously dried over P2 O5) were added 20 ml. of dimethoxymethane and 10.0 g. of phosphorus pentoxide. After one hour, the chloroform solution was decanted and stirred with a sodium carbonate solution, the final product 3,4-Dihydro-2H-pyran obtained.[1]
3,4-Dihydro-2H-pyran as Intermediates
Scientists present a mild and efficient strategy for the synthesis of tetrahydronaphthalenes which was inspired by mechanistic insights obtained during our previous studies towards the synthesis of 3,4-dihydro-2H-pyrans from aryl ketones bearing α-carbonyl substituents. They have developed a mild and catalytic method for the synthesis of tetrahydronaphthalenes bearing a variety of electronically distinct substituents. Mechanistic investigations suggest the role of intermediate 3,4-dihydro-2H-pyrans in the FeCl3-catalyzed synthesis of tetrahydronaphthalenes. This transformation illustrates the ability of aryl ketones to be selectively converted into 3,4-dihydro-2H-pyran or tetrahydronaphthalene products by the use of either a Brønsted or Lewis acid catalyst.[2]
The fragmentation mechanisms of 3,4-dihydro-2H-pyran
Apart from the electron-induced mass spectrometric investigation deciphering primary reaction mechanisms in 29 cyclic ethers important in low-temperature combustion performed by Koritzke et al., the measurements of fragmentation of 3,4-dihydro-2H-pyran by electron impact are non-existent. In the mentioned study, the mass spectrum of 3,4-Dihydro-2H-pyran was only measured at 70 eV, and the processes leading to the formation of the most intensive m/z = 55 and 39 peaks were suggested. Furthermore, on the basis of experimental data on the fragmentation of these ethers, especially oxanes, combined with an analysis of various theoretical models, Koritzke et al. proposed oxygen O(1) as the site most susceptible to electron removal. It was also suggested that the carbon site C(3) is prone to ionization. The parent cations of 3,4-Dihydro-2H-pyran may then undergo two major decomposition pathways. A transannular cleavage of O(1)–C(6) and C(2)=C(3) occurring across the DHP ring in a single step may lead to the production of the m/z = 55 fragment. The second pathway may start from inductive cleavage (initiated from the charge site), followed by the open ring structure rearrangement and the allene (corresponding to the m/z = 39 cation) detachment via the radical-site initiated fragmentation (i.e., α-cleavage). The dissociative ionization of 3,4-dihydro-2H-pyran was studied using electron-induced mass spectrometry combined with molecular dynamics and machine learning simulations. Using a quadrupole mass spectrometer, we recorded the mass spectra in the m/z = 10–90 mass range for 8–140 eV electron energies. These spectra revealed 41 mass peaks that could be assigned to particular cations using theoretical molecular dynamics calculations.[3]
Guided by these calculations and in conjunction with other dissociation measurements, the fragmentation mechanisms were suggested. Generally, the fragmentation pathways of 3,4-Dihydro-2H-pyran observed along the MD trajectories are rather complex and do not seem to exhibit readily recognizable or systematic patterns. The mechanisms typically involve recurrent bond breakings and formations and a sequence of rearrangements before the fragments definitively split. In particular, the electron-induced fragmentation mechanisms of DHP depend on the site at which the molecule is ionized. Two sites are mainly prone to electron attack: oxygen O(1) and carbon C(3). When DHP is ionized at the O(1) site, the O(1)–C(6) bond breaks as the first one, followed by the C(5)–C(6), C(4)–C(5), and C(3)–C(4) bonds breaking, depending on how massive the fragment is formed and accompanied by charge and/or hydrogen migrations. This process may compete with the transannular cleavage mechanism, which starts from ionization at the C(3) site, followed by the initial cleavage of the O(1)–C(6) bond and one of the two bonds between C(2) and C(3). There is an inversion between these processes for most mass groups. This inversion of the fragmentation mechanisms remains somewhat puzzling at the moment, but our results clearly show that it happens.
Scientists have also determined the total and partial ionization cross sections of 37 cations in the energy range from their respective appearance energies (ETH) to 140 eV. To complete the above-mentioned results, we have performed machine learning simulations predicting total ionization cross sections. Comparing the simulation data with the experimental values showed a satisfactory agreement, similar to previous predictions made using the ML algorithm.The measured cross sections show that the production of parent cation C5H8O+ starts at 8.76 (0.02) eV, and it governs the dissociative ionization of 3,4-Dihydro-2H-pyran at lower energies. However, above 18 eV, the m/z = 55 cation becomes the primary fragmentation product. The present results can shed light on the fragmentation of cyclic ether intermediates involved in degenerate chain branching in low-temperature hydrocarbon oxidation and biofuel combustion. Moreover, these results may be used as the input data in radiation-induced damage models to improve radiation treatment protocols. Indeed, the electron-induced ionization cross sections of 3,4-Dihydro-2H-pyran are higher than those measured for similar six-membered heterocyclic molecules containing nitrogen atoms. MD calculations suggest that DHP decomposes into many reactive oxygen-containing fragments with high cross sections. This could pave the way for producing efficient oxygen-rich radiosensitizers containing 3,4-Dihydro-2H-pyran rings. The reactive oxygen species formed after the irradiation of such drugs may alter cancer cells more severely, thereby increasing their therapeutic potential.
References
[1] PLR IP HOLDINGS - US4195180, 1980, A
[2] Watson RB, Schindler CS. Iron-Catalyzed Synthesis of Tetrahydronaphthalenes via 3,4-Dihydro-2H-pyran Intermediates. Org Lett. 2018 Jan 5;20(1):68-71.
[3] Wasowicz TJ, Jurkowski MK, Harris AL, Ljubi? I. Unveiling the electron-induced ionization cross sections and fragmentation mechanisms of 3,4-dihydro-2H-pyran. J Chem Phys. 2024 Aug 14;161(6):064304.
You may like
Related articles And Qustion
See also
Lastest Price from 3,4-Dihydro-2H-pyran manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 110-87-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00/kg2025-09-24
- CAS:
- 110-87-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs