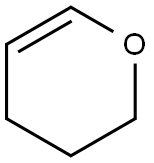
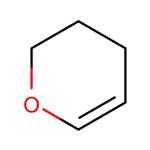
Organic synthesis intermediates:3,4-Dihydro-2H-pyran
General description
3,4-Dihydro-2H-pyran is a natural product found in Gossypium hirsutum with data available. Dihydro-2h-pyran appears as a clear colorless liquid with an ethereal odor. Less dense than water. Vapors heavier than air. Ring conformations of 3,4-dihydro-2H-pyran (34DHP) have attracted considerable interest owing to their structural similarity to cyclohexene, an important molecule in stereochemistry
Chemical structure analysis
Conformational potential energy surfaces and cationic structure of 3,4-dihydro-2H-pyran by VUV-MATI spectroscopy and Franck–Condon fitting:3,4-Dihydro-2H-pyran (34DHP) is a pseudo-five-membered heterocyclic compound obtained by replacing thea-methylene group in cyclohexene with an oxygen atom. Numerous experimental and theoretical studies have compared its conformational structure with that of cyclohexene, one of the most important molecules in stereochemistry. The energies associated with the conformational changes in cyclohexene have been a long-standing conundrum; thus, a key question of how the heteroatom substitution of the methylene group in cyclohexene changes the conformational PES of cyclohexene remains to be answered. Many infrared (IR) and Raman spectroscopic studies on the conformational structure of 34DHP have concluded that the twisted conformer (half-chair form) is the most stable structure, whereas the bent conformation lies at the saddle point for the interconversion between two equivalent twisted conformers (despite the controversy over the possibility of a metastable bent form). In their far-IR spectroscopy analysis, Uedaet al. reported that the interconversion barrier of the twisted conformers through the metastable bent form is 2430 cm based on the 2D PES associated with the conformational interconversion[1].
Application and Pharmacology
1.under Lewis acidic conditions pyran40forms initially. However, the reversibility of this step ultimately leads to the formation of tetrahydronaph-thalene following Friedel−Crafts alkylation and rearomatization. In comparison, Brønsted acid catalysis does not mediate the desired reaction pathway and provides pyrans as products. In summary, a mild and catalytic method for the synthesis of tetrahydronaphthalenes bearing a variety of electronically distinct substituents. Mechanistic investigations suggest the role of intermediate 3,4-dihydro-2H-pyrans in the FeCl3-catalyzed synthesis of tetrahydronaphthalenes. This trans formation illustrates the ability of aryl ketones to be selectively converted into 3,4-dihydro-2H-pyran or tetrahydronaphthalene products by the use of either a Brønsted or Lewis acid catalyst[2].
Synthesis
A series of 3',4'-dihydro-1'H-spiro(indoline-3,2'-quinolin)-2-ones were prepared by the inverse electron demand aza Diels-Alder reaction (Povarov reaction) of imines derived from isatin and substituted anilines, and the electron rich alkenes trans-isoeugenol and 3,4-dihydro-2H-pyran. These compounds were assessed for in vitro antiplasmodial activity against drug-sensitive and drug-resistant forms of the P. falciparum parasite. Three compounds derived from 3,4-dihydro-2H-pyran and four compounds derived from trans-isoeugenol showed antiplasmodial activity in the low micromolar range against the drug-resistant FCR-3 strain (1.52 – 4.20 µM). Only compounds derived from trans-isoeugenol showed antiplasmodial activity against the drug-sensitive 3D7 strain.
A series of 3',4'-dihydro-1'H-spiro(indoline-3,2'-quinolin)-2-ones by the Povarov reaction of electron rich alkenes and ketimines derived from isatin and assessed them for antiplasmodial activity in vitro. Eight of the compounds prepared displayed antiplasmodial activity in the low micromolar range against either the 3D7 or the FCR-3 strain. Interestingly, compounds derived from 3,4-dihydro-2H-pyran did not show activity against the 3D7 strain but did show activity in the low micromolar range against the FCR-3 strain. Compounds derived from isoeugenol displayed moderate antiplasmodial activity against both drug-resistant and drug sensitive strains of the parasite[3].
Safety and Storage
Exposure of rats to a 3,4-dihydro-2H-pyran (DP) saturated vapour atmosphere statically generated from liquid MDP containing 0.037% AC, caused severe irritancy and death from accumulation of AC vapour. Sparging the impure material with nitrogen gas before atmosphere generation significantly reduced or abolished lethal toxicity.
References
1.Watson R. B. & Schindler C. S., "Iron-Catalyzed Synthesis of Tetrahydronaphthalenes via 3,4-Dihydro-2H-pyran Intermediates," Organic Letters, Vol.20, No.1(2018), pp.68-71.
2.Kang D. W., Yoon D. K. & Kwon C. H., "Conformational potential energy surfaces and cationic structure of 3,4-dihydro-2H -pyran by VUV-MATI spectroscopy and Franck–Condon fitting," Physical Chemistry Chemical Physics, Vol.22, No.47(2020), pp.27673-27680.
3Mathebula B., Butsi K. R. & van Zyl R. L. et al., "Preparation and antiplasmodial activity of 3',4'‐dihydro‐1'H‐spiro (indoline‐3,2'‐quinolin)‐2‐ones," Chemical Biology & Drug Design, Vol.94, No.4(2019), pp.1849-1858.
You may like
Related articles And Qustion
See also
Lastest Price from 3,4-Dihydro-2H-pyran manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 110-87-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00/kg2025-09-24
- CAS:
- 110-87-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs