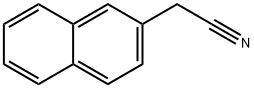
2-Naphthylacetonitrile:Synthesis, Application
General description
2-Naphthylacetonitrile is a white crystalline powder with a melting point of 82-84℃. It is an organic chemical raw material
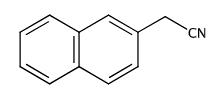
Fig. 1 The structure of 2-Naphthylacetonitrile.
Synthetic routes
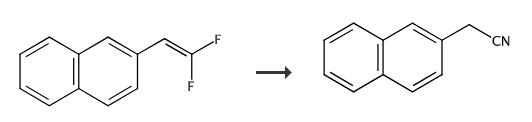
Fig. 2 The synthetic method 1 of 2-Naphthylacetonitrile.
Add gem-difluoro olefin(0.4 mmol, 1 equiv), 28% aqueous ammonia (4 mmol, 10 equiv) and CH3CN (2 mL) to an oven-dried 10mL pressure tube equipped with a stir bar, under air atmosphere. Seal the tube and heat to 60°C (oil bath) for 24 h. Monitor by TLC till completion of the reaction. Flash column chromatography : petroleum ether/EtOAc, 20/1. 1H NMR (400 MHz, CDCl3) δ 7.85.-.7.79(m, 4H), 7.53 (d, J= 5.0 Hz, 2H), 7.35 (d, J = 8.5 Hz, 1H), 3.85 (d, J = 2.6 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 133.3, 132.7, 129.0, 127.8, 127.7, 127.3, 126.82, 126.76, 126.5, 125.5, 117.9, 23.7 [1].
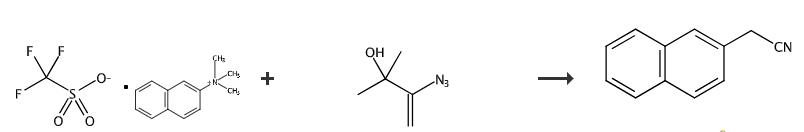
Fig. 3 The synthetic method 2 of 2-Naphthylacetonitrile.
Equip an undivided cell with a reticulated vitreous carbon (RVC) anode (100 PPI, 1 cm × 0.5 cm) and Pt cathode (1 cm × 0.2 cm) and connect to a DC regulated power supply. Add aryl 1, 3-azido-2-methylbut-3-en-2-ol (0.55 mmol), n-Bu4NClO4 and DMF (4 mL) to the cell. Electrolyse the mixture using V = -5.0 V at 30°C under magnetic stirring. Monitor the reaction by TLC. Pour the reaction mixture into diethyl ether (30 mL) and wash with water two times (20 mL × 2). Dry over Na2SO4 and concentrate in vacuo. Purify the residue by column chromatography on silica gel using a mixture of petroleum ether/EtOAc (v : v = 50 : 1). 1H NMR (500 MHz, Chloroform-d) δ 7.87 (dt, J = 9.5, 5.6 Hz, 4H), 7.55 (qd, J = 6.9, 3.5 Hz, 2H), 7.41 (dd, J = 8.4, 2.0 Hz, 1H), 3.93 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 133.4, 132.7, 129.1, 127.8, 127.7, 127.2, 126.9, 126.8, 126.5, 125.5, 117.9, 23.9 [2].
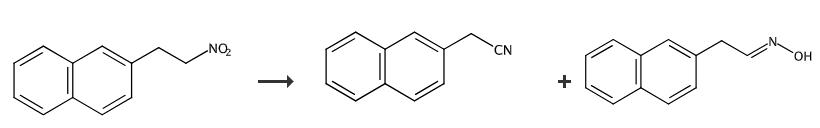
Fig. 4 The synthetic method 3 of 2-Naphthylacetonitrile.
Dissolve the nitro-compound (1 eq.) and N, N-diisopropylethylamine into a portion of the dry dichloromethane (2/3, final concentration 0.25 M) in a round bottomed flask under magnetic stirring and nitrogen atmosphere. Cool the reaction mixture to -20°C and add a solution of HSiCl3 in the remaining solvent dropwise over 2 minutes. Stir for 3 h and quench the reaction mixture with slow addition of a saturated solution of NaHCO3. Separate the aqueous phase and extract 3 times with dichloromethane. Dry the combined organic phases over Na2SO4, filter and evaporate under reduced pressure. Purify the resulting mixture by column chromatography (9:1 hexane/ ethyl acetate) [3].
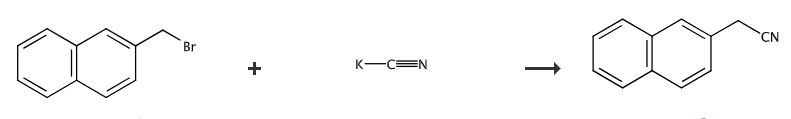
Fig. 4 The synthetic method 4 of 2-Naphthylacetonitrile.
Add 2- (bromomethyl) naphthalene in small portions to a two-necked round-bottomed flask containing a stirring suspension of KCN in DMSO under a flow of N2 gas. Stir at 60°C for 16 h and store overnight at room temperature. Pour the reaction mixture into cold H2O. Filter the solid obtained, wash with cold H2O and dry under reduced pressure [4].
Application
As crystal growth regulators
The rapid growth of perovskite crystals leads to excessive grain boundaries and surface defects, which have a negative effect on the performance of solar cells (PSCs). Passivating defects by controlling the crystal growth rate becomes a crucial research hotspot for preparing high-crystallinity perovskite films. In this work, phenylacetonitrile (PA) and 2-naphthylacetonitrile (2-NA) serving as crystal growth regulators are introduced into the perovskite precursor. The coordination effect of lone-pair electrons (n-electrons) and pi-electrons in the regulator molecule with Pb2+ on the nucleation and growth of FA(0.)(80)MA(0.15)G(0.15)Cs(0.05)Pb(I0.85Br0.15)(3) perovskite crystal along with the passivation of surface defects and grain boundaries are systematically investigated. The pi-electrons of N atom form a coordination bond with Pb', and the Jr-electrons in the aromatic ring generate a cation-pi interaction with Pb2+. This combined effect efficiently delays the crystallization rate of the perovskite crystal and then promotes the grain growth and reduces the grain boundaries, which is favorable for the dissociation of more excitons to carriers. The PA-optimized PSCs shows an increase of power conversion efficiency (PCE) from 18.01 to 21.09%, with an unencapsulated device retaining 91.2% of its initial efficiency for 60 days in 40 +/- 5% relative humidity under dark conditions [5].
References
[1] Zhang J Q, Liu J, Hu D, et al. Rapid and Simple Access to α-(Hetero) arylacetonitriles from Gem-Difluoroalkenes[J]. Organic Letters, 2022, 24(2): 786-790.
[2] Kong X, Wang Y, Chen Y, et al. Cyanation and cyanomethylation of trimethylammonium salts via electrochemical cleavage of C–N bonds[J]. Organic Chemistry Frontiers, 2022, 9(5): 1288-1294.
[3] Pirola M, Faverio C, Orlandi M, et al. Metal‐Free Deoxygenation of Chiral Nitroalkanes: An Easy Entry to α‐Substituted Enantiomerically Enriched Nitriles[J]. Chemistry–A European Journal, 2021, 27(40): 10247-10250.
[4] Yantara N, Jamaludin N F, Febriansyah B, et al. Regulating Vertical Domain Distribution in Ruddlesden–Popper Perovskites for Electroluminescence Devices[J]. The Journal of Physical Chemistry Letters, 2019, 10(24): 7949-7955.
[5] Li J, Dong X, Liu T, et al. Electronic coordination effect of the regulator on perovskite crystal growth and its high-performance solar cells[J]. ACS applied materials & interfaces, 2020, 12(17): 19439-19446.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Naphthylacetonitrile manufacturers

US $9.90/kg2025-04-15
- CAS:
- 7498-57-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $10.00/Kg/Bag2022-06-02
- CAS:
- 7498-57-9
- Min. Order:
- 1Kg/Bag
- Purity:
- 99%
- Supply Ability:
- 20 tons