2-Naphthylacetonitrile: Overview, Pharmaceutical Synthesis Applications and Preparation Method
General Description
2-Naphthylacetonitrile is a versatile compound with a cyano group attached to the beta-position of a naphthalene ring. It is widely used in pharmaceutical synthesis, acting as a c-Myc protein inhibitor with potential applications in treating various diseases. The compound's unique properties make it a valuable candidate for developing novel pharmaceutical compositions targeting central nervous system disorders. The preparation of 2-Naphthylacetonitrile involves a palladium-catalyzed decarboxylative coupling method, resulting in a high yield of 96%. This efficient synthetic route showcases the compound's significance in organic chemistry and its role as a fundamental building block in the synthesis of complex organic molecules. Overall, 2-Naphthylacetonitrile plays a vital role in advancing scientific research and industrial applications, highlighting its importance in various fields such as pharmaceuticals, agrochemicals, and materials science.

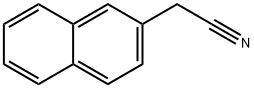
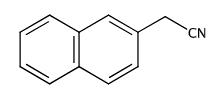
Figure 1. 2-Naphthylacetonitrile
Overview
2-Naphthylacetonitrile, also known as β-Naphthylacetonitrile, is a colorless to pale yellow crystalline solid with a distinct odor. Chemically, it features a cyano group attached to the beta-position of a naphthalene ring, conferring unique properties to this compound. Due to its good chemical stability and solubility in organic solvents, 2-Naphthylacetonitrile is widely used in various applications. The synthesis of 2-Naphthylacetonitrile typically involves the cyanation of 2-naphthylacetic acid or its derivatives. This process allows for the efficient production of this compound, which serves as a crucial intermediate in the synthesis of complex organic molecules. Additionally, 2-Naphthylacetonitrile acts as a fundamental building block in the preparation of functional materials. The versatility of 2-Naphthylacetonitrile makes it indispensable in fields such as pharmaceuticals, agrochemicals, and materials science. Its unique chemical structure enables researchers to explore diverse chemical reactions and develop innovative products. Overall, 2-Naphthylacetonitrile plays a vital role in advancing scientific research and industrial applications, showcasing its significance in the realm of organic chemistry.
Pharmaceutical Synthesis Applications
2-Naphthylacetonitrile has shown promising applications in pharmaceutical reactions, particularly as a c-Myc protein inhibitor. The c-Myc protein inhibitor selectively targets and inhibits the c-Myc protein, making it valuable in the prevention and treatment of diseases associated with c-Myc protein dysregulation, such as cancers, cardiovascular diseases, cerebrovascular diseases, and virus infection-related conditions. By effectively modulating the activity of the c-Myc protein, 2-Naphthylacetonitrile can play a crucial role in combating these serious health issues. Furthermore, 2-Naphthylacetonitrile's properties make it a potential candidate for use in developing novel pharmaceutical compositions aimed at addressing central nervous system disorders. Its unique characteristics and inhibitory effects could contribute to the treatment and prevention of conditions like depression, anxiety, schizophrenia, and sleep disorders. The synthesis and formulation of 2-Naphthylacetonitrile derivatives could pave the way for innovative therapies targeting monoamine reuptake inhibition and modulation of monoamine transporters, offering new avenues for enhancing neurological health and well-being. 1,2
Preparation Method
The preparation of 2-Naphthylacetonitrile involves a specific synthetic method. In this method, the synthesis is achieved through a palladium-catalyzed decarboxylative coupling of cyanoacetate salts with aryl halides and triflates. The reaction utilizes reactants such as 2-Chloronaphthalene and Acetic acid, 2-cyano-, sodium salt, along with catalysts including Di-μ-chlorobis(η3-2-propenyl)dipalladium and 2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl. The detailed procedure involves charging a Schlenk tube with the necessary reactants and catalysts, evacuating the tube, filling it with argon, and adding mesitylene as a solvent. The reaction is then carried out at 140 °C for 5 hours, followed by purification of the residue to obtain the desired product, 2-(naphthalen-2-yl)acetonitrile. The transformation steps after the synthesis include decarboxylation of aliphatic acids and arylation at a carbon containing an active hydrogen, as well as the Hurtley reaction. This synthetic route provides a high yield of 96% for the production of 2-Naphthylacetonitrile, demonstrating the efficiency and effectiveness of the palladium-catalyzed coupling method in obtaining this important organic compound. 3
Reference
1. Tong YZ, Xu R, Chen J, Lai LH. C-myc protein inhibitor, preparation method therefor, and use thereof. 2022; Patent Number: CN114437119.
2. Shao LM, Wang F, Malcolm SC. Cycloalkylamines as monoamine reuptake inhibitors and their preparation, pharmaceutical compositions and use in the treatment of nervous system agents. 2007; Patent Number: WO2007081857.
3. Shang R, Ji DS, Chu L, Fu Y, Liu L. Synthesis of α-aryl nitriles through palladium-catalyzed decarboxylative coupling of cyanoacetate salts with aryl halides and triflates. Angew Chem Int Ed Engl. 2011; 50(19): 4470-4474.
Related articles And Qustion
Lastest Price from 2-Naphthylacetonitrile manufacturers

US $9.90/kg2025-04-15
- CAS:
- 7498-57-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $10.00/Kg/Bag2022-06-02
- CAS:
- 7498-57-9
- Min. Order:
- 1Kg/Bag
- Purity:
- 99%
- Supply Ability:
- 20 tons