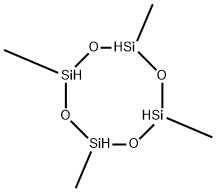
2,4,6,8-Tetramethylcyclotetrasiloxane: An widely used crosslinker
2,4,6,8-Tetramethylcyclotetrasiloxane (Tetramethylcyclotetrasiloxane) is a colorless, volatile liquid used primarily as an intermediate in the production of silicone polymers and resins. It is also used as an impregnant for photoelectric material and to manufacture modified siloxane with defined hydrogen content and chain quantity.

Synthesis of 2,4,6,8-Tetramethylcyclotetrasiloxane
Methyldichlorosilane CH3SICL2 was mixed with a stoichiometric equivalent of water, i. e. 0.5 mole of water per mole of silicon bonded chlorine, in a steam heated continuous hydrolysis reactor. The hydrolysis reactor was maintained at 60 psig and the temperature of the reactor was controlled such that the hydrolyzate exiting the reactor was at a temperature of about 33°C. The hydrolyzate exiting the reactor was analysed by gas chromatography (GC) using a flame ionisation detector (FID) and found to comprise about 95 weight percent linear chlorine end-terminated methylhydrogensiloxane species and about five weight percent cyclic methylhydrogensiloxanes species containing 2,4,6,8-Tetramethylcyclotetrasiloxane. The hydrolyzate was diluted to about 20% IN ISOPAR P'hydrocarbon solvent containing 0. 1% dodecylbenzenesulphonic acid catalyst. Excess HC1 gas from the hydrolysis reaction was collected for re-use.[1]
The diluted hydrolyzate containing catalyst was fed through a simple rearrangement reactor tank at ambient temperature and pressure. Residence time of the diluted hydrolyzate in the reactor was about 3 hours. GC-FID analysis of the product exiting the rearrangement reactor showed the siloxane component to consist of about 70 weight percent linear chlorine end-terminated methylhydrogensiloxane species and about 30 weight percent cyclic methylhydrogensiloxane species containing 2,4,6,8-Tetramethylcyclotetrasiloxane. The product from the rearrangement reactor was heated to drive off aqueous HC1, stripped in flash drums under vacuum and then vacuum distilled and the cyclic species and low-boiling linear species taken overhead. The bottom fraction was cooled and recycled to the rearrangement reactor. Residual chlorine in the overhead fraction from the flash distillation (the crude product) was removed by contact with a calcium carbonate calcium chloride water system. The product was finally dried in a magnesium oxide bed. The recovered cyclic methylhydrogensiloxane fraction was analysed by GC-FID and found to comprise 99.7 weight percent cyclic methylhydrogensiloxanes of the tetramer, pentamer, and hexamer species, which contain 25% 2,4,6,8-Tetramethylcyclotetrasiloxane. The process was run continuously for about 20 days.
Porous Materials Cross-Linked with 2,4,6,8-Tetramethylcyclotetrasiloxane
V3 polymer/ 2,4,6,8-Tetramethylcyclotetrasiloxane porous, monolithic materials (polyHIPEs) were obtained by cross-linking of V3 polymer with 2,4,6,8-Tetramethylcyclotetrasiloxane in water-in-oil (w/o) HIPE conditions in the way described. The continuous phase of HIPE contained the polymer, the cross-linking agent, the surfactant (DBE-224), additional porogen (chlorobenzene), and Karstedt hydrosilylation catalyst, whereas the internal phase was the 0.02M NaCl aqueous solution. The amounts of 2,4,6,8-Tetramethylcyclotetrasiloxane used were such that molar ratios of Si-Vinyl of the polymer to Si-H groups of it in the experiments were equal to 2.25:1, 1.5:1, 1:1, and 1:1.5 for the samples denoted respectively.[2]
Materials studied in this work were prepared by cross-linking of V3 polymer with 2,4,6,8-Tetramethylcyclotetrasiloxane in w/o HIPE. The reactions were carried out at various molar ratios of Si-Vinyl: Si-H groups in order to obtain various V3 polymer cross-linking degrees and various amounts of reactive groups (Si-Vi, Si-H) remaining in the resultant systems. Both these factors seemed to be important for Pd incorporation into the materials as well as for their subsequent ceramization. Swelling experiments showed that (as planned) the samples differed in cross-linking degree. Their swelling decreased—i.e., cross-linking degree increased—as the amount of 2,4,6,8-Tetramethylcyclotetrasiloxane with respect to V3 polymer in the experiment increased. It should be noted that swelling of the 2.25V3_1Si-H sample, that exhibited the lowest cross-linking level, was 1.8-times higher than that of the 1V3_1.5Si-H sample, of the highest cross-linking degree. Moreover, swelling of the prepared materials was significantly higher than that of the networks containing methylvinylsiloxane units obtained in the bulk which indicates that their cross-linking degrees were low. This is because, similarly to the previous studies when V3 polymer cross-linking was conducted in the bulk , participation of all reactive groups of the polymer (with a vinyl group at each Si atom) and 2,4,6,8-Tetramethylcyclotetrasiloxane (with four Si-H groups in the cyclic molecule), due to steric reasons, was improbable. Furthermore, performance of the reactions in HIPE was an additional disadvantage. Polymer cross-linking occurred in thin layers of the continuous phase surrounding high amount of internal phase droplets which must have caused effective contact between the reactive groups difficult.
Polysiloxane networks were prepared by hydrosilylation of poly(methylvinylsiloxane) (V3 polymer) with 1,3,5,7-tetramethylcyclotetrasiloxane (2,4,6,8-Tetramethylcyclotetrasiloxane) at various Si-Vinyl: Si-H groups molar ratios in water-in-oil high internal phase emulsion (HIPE). Curing the emulsions followed by removal of water led to foamed cross-linked polysiloxane systems differing in the cross-linking degrees, as well as residual Si-H and Si-Vinyl group concentrations. Treatment of thus obtained materials in Pd(OAc)2 solution in tetrahydrofuran resulted in the formation of porous palladium/polymer nanocomposites with different Pd contents (1.09–1.70 wt %).
Hydrophobic Modification of Wood Using 2,4,6,8-Tetramethylcyclotetrasiloxane
Hydrophobic surfaces have aroused considerable attention because of their extensive potential applications. In this work, we developed a facile strategy for fabricating hydrophobic and anti-fouling surfaces on wood substrates. The modification was accomplished simply by immerging wood into the 2,4,6,8-Tetramethylcyclotetrasiloxane (D4H) modifier solution for 5 min. The D4H modified wood was characterized using Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscope, and energy dispersive spectrometer. The result shows that the 2,4,6,8-Tetramethylcyclotetrasiloxane modified wood had good hydrophobicity, and the water contact angle of wood in the radial and cross sections reached 140.1° and 152°. In addition, the obtained hydrophobic wood surface also showed anti-fouling properties, UV resistance and could withstand the tape peel test and finger wiping.[3]
The mechanism of 2,4,6,8-Tetramethylcyclotetrasiloxane modification to prepare hydrophobic wood is: the hydrophilic –OH groups on the wood surface will undergo a dehydrogenation reaction with the –Si–H bonds on the D4H structure in the presence of catalysts, so that the D4H with hydrophobic methyl (–CH3) groups will be grafted onto the wood surface to finally obtain hydrophobized wood. 2,4,6,8-Tetramethylcyclotetrasiloxane is a reactive siloxane containing four Si–H participates in various chemical reactions, especially with unsaturated olefins and –OH groups containing materials, such as wood with abundant hydroxyl groups. In addition, the methyl group in the D4H structure makes it have low surface energy. The surface of unmodified wood is composed of a large number of hydrophilic –OH groups. In the presence of a Kastredt catalyst, the –OH groups on the wood surface undergo an ultrafast dehydrogenation reaction with 2,4,6,8-Tetramethylcyclotetrasiloxane. As shown, after immersing the wood samples in the D4H modifier solution, a large number of air bubbles was seen. Through this simple modification method, it can be speculated that D4H is covalently grafted onto the wood surface. Consequently, the resulting wood samples will have good hydrophobic properties.
In summary, highly hydrophobic wood with good anti-fouling properties was successfully fabricated by simple soaking in a D4H modifier solution. The proposed formation mechanism of hydrophobicity on the wood surface resulted from 2,4,6,8-Tetramethylcyclotetrasiloxane with low surface energy covalently grafting on the surface of the wood. The WCA of the D4H treated wood was increased significantly compared to that of untreated wood, and the water absorption of the 2,4,6,8-Tetramethylcyclotetrasiloxane -treated (25% content) wood samples was lower than 40% after 24 h of water immersion. In addition, the obtained hydrophobic wood showed good UV resistance and wear resistance, which indicates promising application prospects in numerous fields.
References
[1]DOW SILICONES - WO2005/5441, 2005, A2
[2]Mrówka J, Partyka J, Hasik M. Porous Materials Based on Poly(methylvinylsiloxane) Cross-Linked with 1,3,5,7-Tetramethylcyclotetrasiloxane in High Internal Phase Emulsion as Precursors to Si-C-O and Si-C-O/Pd Ceramics. Materials (Basel). 2021 Sep 29;14(19):5661.
[3]ang M, Fang X, Li B, Xu M, Wang H, Cai S. Hydrophobic Modification of Wood Using Tetramethylcyclotetrasiloxane. Polymers (Basel). 2022 May 19;14(10):2077.
Lastest Price from 2,4,6,8-Tetramethylcyclotetrasiloxane manufacturers

US $110.00-70.00/kg2025-04-21
- CAS:
- 2370-88-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000
US $0.00-0.00/kg2025-04-18
- CAS:
- 2370-88-9
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 97%
- Supply Ability:
- 200tons