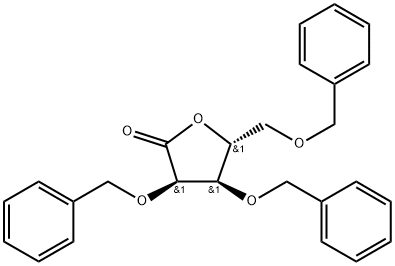
2,3,5-Tri-O-benzyl-D-ribonolactone: applications in organic synthesis and safety
General Description
2,3,5-Tri-O-benzyl-D-ribonolactone is a compound used in organic synthesis, particularly in the synthesis of remdesivir and 4-deazaformycin A. It plays a crucial role in the production of these important drugs. However, it is important to note that 2,3,5-Tri-O-benzyl-D-ribonolactone is classified as an acute toxic substance and requires careful handling and adherence to safety precautions. Overall, its versatile applications in chemical synthesis highlight its significance in the development of lifesaving medications and novel therapeutics, but caution must be exercised to minimize potential harm.

Figure 1. 2,3,5-Tri-O-benzyl-D-ribonolactone
Applications in organic synthesis
Synthesis of remdesivir
2,3,5-Tri-O-benzyl-D-ribonolactone is a compound that has been primarily used as a key intermediate in the synthesis of remdesivir, which is the first and only FDA-approved antiviral drug for COVID-19 treatment. The compound is synthesized using a Weinreb amide approach, which involves three sequential steps starting from 2,3,5-tri-O-benzyl-d-ribonolactone. This approach enables a high-yield (65%) synthesis of the intermediate at a large scale. The use of this compound in the production of remdesivir is particularly crucial given the current COVID-19 pandemic, as adequate supplies of the drug are highly warranted to cope with this global public health crisis. The scalable production of the key remdesivir intermediate using this approach is expected to contribute to meeting the increasing demand for the drug, ensuring its availability to those who need it the most. Overall, 2,3,5-Tri-O-benzyl-D-ribonolactone plays an important role in the production of remdesivir and its use highlights the significance of chemical synthesis in the development of lifesaving medications. 1
Synthesis of 4-deazaformycin A
2,3,5-Tri-O-benzyl-D-ribonolactone is a compound that has broad applications in chemical synthesis, including the preparation of 4-deazaformycin A. The synthesis of 4-deazaformycin A involves several steps, with the first key step being the condensation of suitably substituted, lithiated 4-picoline with 2,3,5-tri-O-benzyl-d-ribonolactone. This intermediate undergoes dehydration to form a hemiacetal, which is then subjected to ionic hydrogenation. The protecting groups are then manipulated, and the resulting compound is used in a ring-closing reaction to give 7-amino-3-(beta-d-ribofuranosyl)pyrazolo[3,4-c]pyridine, which is an important intermediate in the synthesis of 4-deazaformycin A. 2,3,5-Tri-O-benzyl-D-ribonolactone plays a crucial role in the synthesis of 4-deazaformycin A, which is a natural product with potential anticancer activity. The compound's application in this synthesis highlights its importance in chemical synthesis and underscores the significance of chemical synthesis in the discovery and development of novel therapeutics. In conclusion, the use of 2,3,5-Tri-O-benzyl-D-ribonolactone in the synthesis of 4-deazaformycin A underscores its versatile applications in chemical synthesis and its potential contribution to the development of important therapeutics. 2
Safety
2,3,5-Tri-O-benzyl-D-ribonolactone has been classified as an acute toxic substance. It falls under category 4 of oral, dermal, and inhalation toxicity, which indicates that it may cause harm upon ingestion, skin contact, or inhalation even in small quantities. The warning label and hazard statements also emphasize the harmful effects of this substance. To ensure safe handling and use of 2,3,5-Tri-O-benzyl-D-ribonolactone, certain precautionary measures should be observed. These include wearing protective gloves, clothing, eye protection, face protection, and hearing protection when handling the substance. It is also advised to work outdoors or in a well-ventilated area to avoid breathing in dust, fume, gas, mist, vapors, or spray. After handling the substance, thorough washing is required to avoid potential exposure. In case of accidental exposure, specific treatments should be administered based on the label instructions. If swallowed, medical help should be sought immediately, and the mouth should be rinsed. In case of skin contact, the affected area should be washed with plenty of water, contaminated clothing should be removed and washed before reuse. If inhaled, the person should be moved to an area with fresh air and kept comfortable for breathing. Overall, it is important to handle 2,3,5-Tri-O-benzyl-D-ribonolactone with great care due to its acute toxicity, following all the necessary safety precautions and adhering to the label instructions to minimize any potential harm. 3
Reference
1. Xie Y, Hu T, Zhang Y, Wei D, Zheng W, Zhu F, Tian G, Aisa HA, Shen J. Weinreb Amide Approach to the Practical Synthesis of a Key Remdesivir Intermediate. J Org Chem. 2021 Apr 2;86(7):5065-5072.
2. Kourafalos VN, Marakos P, Pouli N, Townsend LB. The synthesis of 4-deazaformycin A. J Org Chem. 2003 Aug 8;68(16):6466-6469.
3. Chemical Safety Data Sheet MSDS/SDS: 2,3,5-Tri-O-benzyl-D-ribonolactone. ChemicalBook, 2023.
You may like
Related articles And Qustion
See also
Lastest Price from 2,3,5-Tri-O-benzyl-D-ribonolactone manufacturers

US $0.00/kg2025-09-26
- CAS:
- 55094-52-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00-0.00/KG2025-04-21
- CAS:
- 55094-52-5
- Min. Order:
- 100g
- Purity:
- 98%min HPLC
- Supply Ability:
- 100kg