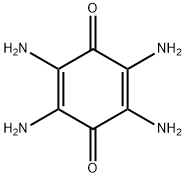
2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione:Application And Chemical Studies
General Description
The 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione is an important intermediate product, in particular for the synthesis of drugs. For example, the 2.5-dialkoxycarbonyl-3.6-diamino benzoquinones which can be prepared from this product by reaction with chloroform esters are active against coccidiosis.[1]It is soluble in N-Methyl-2-pyrrolidone (NMP), and it undergoes a dissolution-reprecipitation process during electrode manufacturing.[2]

Figure 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione powder
Preparation
One side,2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione was obtained by a two-step synthesis with low-cost reactants based on the some reserach.It was prepared according to a previously reported method64 as summarized in the following steps and described below.
Step 1:20.0 g (0.081 mol) of 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione, 60.0 g (0.32 mol) of potassium phthalimide and 200.0 mL of acetonitrile were added into a 500 mL round bottom flask. The mixture was kept at 80 °C for 12 h under stirring. After cooling down to room temperature, the solid was separated by filtration and washed with 2 L boiled water. The product was dried at 60 °C overnight, and brown-yellow powder of tetra (phthalimido)-benzoquinone (TPQ) was obtained (45.0 g, yield = 80.7%).
Step 2:9.41 g (0.014mol) of TPQ was dispersed in 200mL of 80% hydrazine hydrate in a 500mL round bottom flask. The mixture was stirred at room temperature for 2 h, followed by heating at 65 °C for 2 h. The systemwas cooled down to room temperature, filtered, washed with water and ethanol, and the dark purple product of it was obtained (1.3 g, yield= 56.6%). [2] 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dionesynthetic routes is as follow:
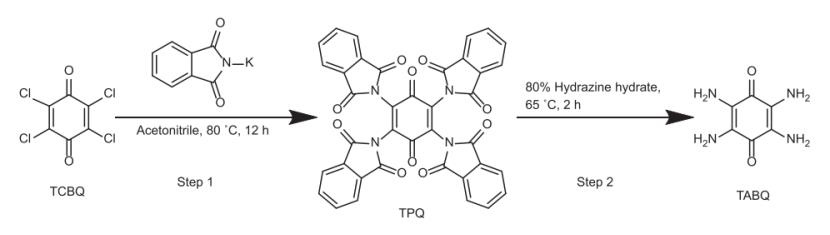
Figure2 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione synthetic routes
What’s more, 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione was prepared using the method of Wallenfels and Draber in a yield of 81%, mp>320 °C (lit. decomposes at 260 °C).[4]
Application
On the one hand, researchers use a symmetric small molecule quinone cathode, 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione, with desirable functional groups to protonate and accomplish dominated proton insertion from weakly acidic zinc electrolyte. The hydrogen bonding network formed with carbonyl and amino groups on the 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione, 2,3,5,6-tetraamino- molecules allows facile proton conduction through the Grotthusstype mechanism. It guarantees activation energies below 300 meV for charge transfer and proton diffusion. The 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione cathode delivers a high capacity of 303 mAh g−1 at 0.1 A g−1 in a zinc-organic battery. With the increase of current density to 5 A g−1, 213 mAh g−1 capacity is still preserved with stable cycling for 1000 times.
Considering the previous factors, we herein specifically apply a symmetric small molecule of 2,5-Cyclohexadiene-1,4-dione, 2,3,5,6-tetraamino- as the cathode material for aqueous zinc-organic batteries. The four amino groups are available for protonation in weakly acidic zinc electrolytes, which would create a proton active environment and initiate proton insertion. At the same time, hydrogen bonds are formed among amino and carbonyl groups, so that the compound does not sublimate even at elevated temperatures. More importantly, the hydrogen bonding network allows facile proton conduction through the breaking and reforming of hydrogen bonds. The2,5-Cyclohexadiene-1,4-dione, 2,3,5,6-tetraamino- electrode thus functions with dominated proton de/ intercalation and experiences low activation energies for charge transfer and diffusion. Excellent electrochemical performance is achieved.[2]
On the other hand, The 2,3,5,6-tetraaminocyclohexa-2,5-diene-1,4-dione can be used to synthetize the p-Benzoquinone [2,3-b:2,3-b]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile]3. The condensation reaction of 2,5-Cyclohexadiene-1,4-dione, 2,3,5,6-tetraamino- 1 with 4,5-Dichloro3,6-dihydroxy-phthalonitrile 2 yielded the tetranitrile derivative, p-benzoquinone [2,3b:2,3-b]bis[(5,8-dihydroxybenzopyrazine)-6,7-dinitrile] 3 For 6 h, a combination of 2,5-Cyclohexadiene-1,4-dione, 2,3,5,6-tetraamino-1 (0.01 mol) and bimolar ratio of 4,5-Dichloro-3,6-dihydroxy-phthalonitrile 2(0.02 mol) was refluxed in ethyl alcohol (50 mL)/pyridine (20 mL) mixture. At the end of the refluxing, the mixture had turned a deep blue color. It was filtered while heating, precipitated with cold water, washed multiple times with distilled water, dried, and crystallized using ethyl alcohol. Yield: 94%. [3]
Reference
[1]Farbwerke Hoechst A.-G.2,3,5,6-Tetraamino-1,4-benzoquinone[P].FR1381640.1964, 12.11.
[2]Lin Z, Shi H Y, Lin L, et al. A high capacity small molecule quinone cathode for rechargeable aqueous zinc-organic batteries[J]. Nature Communications. 2021, 12(1): 1-9.
[3]Al-Warhi T, Abualnaja M, Abu Ali O A, et al. Metallic Porphyrazine Networks: Synthesis, as Well as Thermal and Optical Properties for Accelerating the Oxidation of Thiols to Their Disulfides[J]. Metals. 2022, 12(9): 1523.
[4]Wallenfels K, Draber W. über Fluorchinone. Die nucleophile Substitution mit Aminen[J]. Justus Liebigs Annalen der Chemie.1963, 667(1): 55-71.