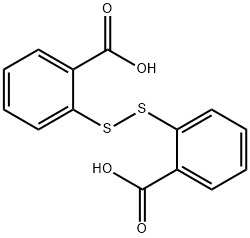
2,2'-Dithiosalicylic acid: Synthesis and Identification
2,2'-Dithiosalicylic Acid is used as an intermediate for the synthesis of pharmaceuticals, dyes, photochemicals (thioxanthones) and biocides (proxel). It is a brown powder that can be biodegraded into transient metabolites such as benzoic acid. As a sulfhydryl modifying reagent, it has the ability to cocrystallize with various compounds, forming organic salts and cocrystals.

Synthesis of 2,2'-Dithiosalicylic acid
[Carboxyl-14C]anthranilic acid 2 with a specific activity of200 MBq mmol-1 was prepared according to previous report. To an aqueous solution of [carboxyl-14C]anthranilic acid (685 mg, 1000 MBq, 5 mmol) in water (5 mL) and concentrated hydrochloric acid(1 mL) at 5°C, a solution of sodium nitrite (345 mg) in water (2.5 mL) was added at such a rate as to keep the temperature below 5°C. The resulting solution of the diazonium derivative was slowly added to the alkaline sodium disulfide solution, keeping the temperature below 5°C. Then, the mixture was allowed to warm up to room temperature. After the evolution of nitrogen ceased, a solution of concentrated hydrochloric acid (0.9 mL) was added. Then, the resulting precipitate of 2,2'-Dithiosalicylic acid was filtered off and washed with water. After that, the precipitate was dissolved in a solution of anhydrous sodium carbonate(300 mg) in water (10 mL) by heating, and the mixture was filtered while hot. Finally, the 2,2'-Dithiosalicylic acid was reprecipitated by adding concentrated hydrochloric acid. The resulting product was filtered off, washed with cooled water and ether, and dried under vacuum to give 3 (655 mg, 850 MBq,2.14 mmol) in 86% yield. IR (KBr): 3100, 2600, 1675,1250, 1450 cm-1.[1]
Three New Polymorphs of 2,2'-Dithiosalicylic acid
Polymorphism is the ability of a solid material to exist in more than one form or crystal structure and this is of interest in the fields of crystal engineering and solid-state chemistry. 2,2'-(Disulfanediyl)dibenzoic acid (also called 2,2'-dithiosalicylic acid, DTSA) is able to form different hydrogen bonds using its carboxyl groups. The central bridging S atoms allow the two terminal arene rings to rotate freely to generate various hydrogen-bonded linking modes. DTSA can act as a potential host molecule with suitable guest molecules to develop new inclusion compounds. We report here the crystal structures of three new polymorphs of the inclusion compound of 2,2'-Dithiosalicylic acid and trimethylamine, namely trimethylazanium 2-[(2-carboxyphenyl)disulfanyl]benzoate 2,2'-(disulfanediyl)dibenzoic acid monosolvate, C3H10N·C+14H9O4S2-·C14H10O4S2, (1), tetrakis(trimethylazanium) bis{2-[(2-carboxyphenyl)disulfanyl]benzoate} 2,2'-(disulfanediyl)dibenzoate 2,2'-(disulfanediyl)dibenzoic acid monosolvate, 4C3H10N·2C+14H9O4S2-·C14H8O4S22-·C14H10O4S2, (2), and trimethylazanium 2-[(2-carboxyphenyl)disulfanyl]benzoate, C3H10N·C+14H9O4S2-, (3). In the three polymorphs, 2,2'-Dithiosalicylic acid utilizes its carboxyl groups to form conventional O-H...O hydrogen bonds to generate different host lattices. The central N atoms of the guest amine molecules accept H atoms from 2,2'-Dithiosalicylic acid molecules to give the corresponding cations, which act as counter-ions to produce the stable crystal structures via N-H...O hydrogen bonding between the host acid and the guest molecule. It is noticeable that although these three compounds are composed of the same components, the final crystal structures are totally different due to the various configurations of the host acid, the number of guest molecules and the inducer (i.e. ancillary experimental acid).[2]
Identification of Disulfides from the Biodegradation of Dibenzothiophene
Two sequential abiotic, net losses of both a carbon and an oxygen atom produced two additional disulfides, 2-oxo-2-(2-thiophenyl)ethanoic acid 2-benzoic acid disulfide and 2,2′-dithiosalicylic acid. To date, there is no proof that 2-mercaptobenzoic acid is formed directly from the 2,3-dione and not from a concurrent metabolic pathway. Finkel'stein et al. also identified 2,2'-Dithiosalicylic acid in dibenzothiophene-degrading culture and suggested that it was a product of 2-mercaptobenzoic acid oxidation forming the disulfide. Other than 2,2'-Dithiosalicylic acid, no other disulfides have been reported as products of dibenzothiophene biodegradation.[3]
The frequency with which 2,3-diones have been detected in acidified extracts of cultures degrading benzothiophenes and dibenzothiophenes prompted us to attempt to study the biodegradation of benzothiophene-2,3-dione. However, analytical difficulties were encountered even while monitoring the sterile controls, suggesting that abiotic reactions were occurring which complicated interpretation of the results. The observed abiotic reactions of HFBT in dibenzothiophene-degrading cultures, caused us to investigate whether 2-mercaptophenylglyoxylate could undergo abiotic reactions, leading to highly polar organic compounds such as 2,2'-Dithiosalicylic acid. Development of analytical methods to detect these highly polar compounds allowed us to examine the culture supernatant of a dibenzothiophene-degrading bacterium for their presence. The understanding gained from studying the abiotic processes helped to explain the presence of two novel disulfides that are dicarboxylic acids similar to 2,2'-Dithiosalicylic acid.
The major goals of bioremediation are the removal of contaminants and the reduction of toxicity in a contaminated area. The formation of products that are more toxic than the original contaminates is undesirable. No information on the toxicity of compounds C and D could be found. However, 2,2'-Dithiosalicylic acid, the final disulfide in the reaction series , is likely not very toxic to humans because its magnesium salt was clinically compared with aspirin as a medication for patients with rheumatoid arthritis. 2,2'-Dithiosalicylic acid has also been detected as a decomposition product of thimerosal, which is widely used in pharmaceutical products such as eye drops, in which it serves as an antibacterial and antifungal preservative. Although 2,2'-Dithiosalicylic acid has antimicrobial properties, we have observed that it can serve as the sole carbon and sulfur source in aerobic soil enrichment cultures (this will be the topic of a future paper). Thus, it is possible that the other two disulfides (compounds C and D) may be biodegraded. If these activities can be detected, it should be possible to assemble a microbial consortium to mineralize dibenzothiophene via the Kodama pathway. The abiotic reactions in the lower portion will provide the substrates for the disulfide-degrading population, and dibenzothiophene would be mineralized to carbon dioxide and sulfate. To date, there have been no reports on the mineralization of dibenzothiophene via the Kodama pathway. The experimental approach described above may demonstrate this mineralization
References
[1] Saadatjoo; Javaheri; Saemian; Amini[Radiochemistry, 2016, vol. 58, # 5, p. 528 - 531] [Radiokhimiya, ;2016, vol. 58, # 5, p. 455 - 457,3]
[2] Yang Y, Li L, Zhang L, Dong W, Ding K. Three polymorphs of an inclusion compound of 2,2'-(disulfanediyl)dibenzoic acid and trimethylamine. Acta Crystallogr C Struct Chem. 2016 Dec 1;72(Pt 12):981-989.
[3] Bressler DC, Fedorak PM. Identification of disulfides from the biodegradation of dibenzothiophene. Appl Environ Microbiol. 2001 Nov;67(11):5084-93.
See also
Lastest Price from 2,2'-Dithiosalicylic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 119-80-2
- Min. Order:
- 1KG
- Purity:
- 96.0%
- Supply Ability:
- 1000kg/month
US $10.00/kg2025-04-21
- CAS:
- 119-80-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 TON