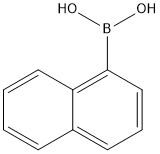
1-Naphthylboronic Acid: Basic Properties and Applications
1-Naphthylboronic Acid is an important compound in the field of organic synthesis, its CAS No. is 13922-41-3, molecular formula is C10H9BO2, molecular weight is 171.99 g/mol, the appearance of off-white to pinkish-beige powder, with a 208 -214 °C melting point, 381.9 ± 25.0 °C boiling point and 1.21 ± 0.1 g/ml density. It has a melting point of 208.9 -214 °C, a boiling point of 381.9 ± 25.0 °C, and a density of 1.21 ± 0.1 g/ml. 1-Naphthylboronic Acid is soluble in methanol but insoluble in water, and structurally it has a boronic acid group attached to the 1-position of the naphthalene ring B(OH) 2; caution is required in its use. Avoid contact with skin, eyes, clothing, inhalation or accidental ingestion, wear gloves, goggles and other personal protective equipment during operation, refer to the Safety Data Sheet (SDS) provided by the supplier for detailed safety information, store in a cool, dry environment with tightly closed containers, stored at room temperature, and keep away from oxidizing agents; in practice, it is a key reagent in organic synthesis. In practical applications, 1-Naphthylboronic Acid is a key reagent in organic synthesis, especially in Suzuki coupling (Suzuki coupling) reaction to play an important role, but also can be used for the synthesis of potassium aryl trifluoroborate (these substances are aryl boron difluoride Lewis acid precursor), but also involved in the preparation of polycyclic aromatic hydrocarbons, and in the recommended storage conditions of the nature of the stability of the oxidizing agent with the existence of incompatibility.

Synthesis of Axially Chiral Biaryls using 1-Naphthylboronic acid
A number of synthetic approaches to this motif have been explored including metal-mediated diastereoselective reactions of substrates with chiral tethering units, auxiliaries, or leaving groups. Enantioselective metal-catalyzed reactions including the oxidative dimerization of phenols and metal-catalyzed [2 + 2 + 2] formal cycloadditions have also been reported. Herein we report our efforts to expand the substrate scope of our previously reported method. Further, we describe combined experimental and computational studies that attempt to rationalize the observed selectivities. DFT calculations indicate that the combination of hydrogen-bonding interactions and steric interactions between the addends and the ligand are responsible for stereoinduction during the reductive elimination step of these reactions. We initiated these investigations by examining reactions of 2-methyl-1-naphthylboronic acid with aryl halide coupling partners incorporating ester, phosphonate, and phosphine oxide substituents in the ortho position. Products arising from ester-functionalized aryl halides were obtained in greater than 80% yield but showed only moderate ee values (40−45%).[1]
Because of the ease of removal of the cumyl protecting group to reveal the free primary amide for further synthetic manipulation and the high selectivity of these reactions (vide infra), we elected to further explore the scope of reactions involving these types of substrates. We summarize results obtained upon coupling of cumyl amide-substituted aryl halides with 1-Naphthylboronic acid. Products are obtained in good to excellent yields and with ee values typically being 88−94%.n summary, this manuscript discloses the enantioselective syntheses of axially chiral biaryl amides by Suzuki−Miyaura reactions catalyzed by the combination of Pd(OAc)2. These reactions typically form compounds in yields greater than 80% and typical enantioselectivities range from 80 to 94%. Chloro-, bromo-, and iodophenylamides form products with similar yields and selectivities. Both electron-rich and electron-poor phenylamides are well tolerated in reactions with 1-Naphthylboronic acid. The stereochemistry of the products arising from the transition states is greatly influenced by the direction in which the tolyl addend twists and the relative energies of closely related transition state analogues are therefore dependent on the interaction of methyl or proton substituents on the tolyl addend with the phosphonate substituent.
References
[1]Shen, X., Jones, G. O., Watson, D. A., Bhayana, B., & Buchwald, S. L. (2010). Enantioselective synthesis of axially chiral biaryls by the Pd-catalyzed Suzuki-Miyaura reaction: Substrate scope and quantum mechanical investigations. Journal of the American Chemical Society, 132(32), 11278–11287. https://doi.org/10.1021/ja104297g
Lastest Price from 1-Naphthylboronic acid manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 13922-41-3
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
US $5.00-1.00/KG2024-03-25
- CAS:
- 13922-41-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available