2-Formylbenzenesulfonic Acid Sodium Salt: Properties and Schiff Base-Based Applications
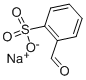
2-Formylbenzenesulfonic acid sodium salt is an important aromatic organosulfonate, the chemical formula is C ₇ H ₅ NaO ₄ S, molecular weight 208.17. Its molecular structure contains both aldehyde group (-CHO) and sodium sulfonate group (-SO₃Na). Its molecular structure contains both aldehyde group (-CHO) and sodium sulfonate group (-SO₃Na), this unique bifunctional structure gives it excellent chemical reactivity and a wide range of industrial applications. 2-Formylbenzenesulfonic acid sodium salt is white to beige crystalline powder, easily soluble in water (1g/10 mL), forming a clear colorless to light brown-yellow solution. Its melting point is 250-255°C (decomposition), density about 1.48 g/cm³, chemically stable at room temperature and pressure, but need to avoid contact with strong oxidizing agents. The presence of aldehyde group in the molecule gives it typical aldehyde reactivity (e.g. oxidation, condensation), while the sodium sulfonate group gives it good water solubility and surface activity, which makes it show unique advantages in the field of dyestuffs and detergents. With the tightening of environmental regulations and the development of green chemistry technology, the market demand for 2-Formylbenzenesulfonic acid sodium salt continues to grow. the market size in China reached hundreds of millions of dollars in 2024, and is expected to expand at a CAGR of about 10% from 2025-2030, mainly driven by the upgrading of the demand in the fields of fluorescent whiteners, pharmaceuticals, and electronic chemicals. In the future, the development of more efficient catalytic systems and green synthesis processes will become a hot research topic in the industry to further improve product performance and reduce environmental impact.

Optical properties of 2-formylbenzenesulfonic acid sodium salt-based Schiff bases
2-Formylbenzenesulfonic acid has many uses. These are mainly; it is used as fluorescent brighteners in special coloring applications, to give a bright blue to plastics, coatings and products such as detergents, as an additive in electroplating processes and the manufacture of special textiles. The 2-Formylbenzenesulfonic acid sodium salt is a polar molecule that can be used as an intermediate in chemical syntheses. The electron-withdrawing sulfoxide group attracts the electron density towards itself, polarizing the benzene ring and making it suitable for electrophilic addition reactions. Therefore, 2-formylbenzenesulfonic acid sodium salt is a very suitable reagent in the synthesis of special chemicals and intermediates. Aminophenols are synthesis precursors, and their derivatives have important uses in both the photographic, and pharmaceutical industries. They are used as precursors and intermediates in the synthesis of complex molecules, especially in the dyeing and dye industry. Schiff base and metal complexes were synthesized from the reactions of 2-aminophenol and various aldehydes and their biological activities were studied.[1]
Sodium 2-formylbenzenesulfonate (0.45 g, 2.18 × 10−3 mol) was added to EtOH (100 mL) solution of 2-amino-4-chlorophenol (0.31 g, 2.18 × 10−3 mol). The mixture was stirred and refluxed for 1 h. The compound was obtained from the evaporation of EtOH. It was crystallized from CHCl3: n-hexane (3:2) as a yellow solid, m.p. 206–208 °C, 0.61 g (85%) yield. Colorimetric and spectroscopic studies were performed according to references by preparing 50 µM solutions of compounds 1 and 2, and tetrabutylammonium salts in DMSO.In this study, 2-formylbenzenesulfonic acid sodium salt-based Schiff bases were synthesized from the reactions of 2-formylbenzenesulfonic acid sodium salt with 2-amino-4-chlorophenol and 2-amino-4-methylphenol. The structure of the compounds was determined by FTIR, UV–Vis and NMR spectroscopy methods using both experimental and DFT methods. The anion sensor properties of the compounds were investigated both UV–Vis spectroscopically and calorimetrically. In addition, the antimicrobial activities, interactions with DNA, DNA cleavage and antioxidant activities of Schiff bases were investigated. Additionally, the effects of group electronegativity on the spectroscopic, biological, chromogenic sensing and optical properties of chlorine and methyl side groups and sodium 2-sulfobenzalidene-aminophenol-based Schiff bases were investigated.
References
[1]Yıldız, E. A., Pepe, Y., Erdener, D., Karatay, A., Boyacıoğlu, B., Ünver, H., Yapar, G., Demir, N., Yıldız, M., & Elmalı, A. (2023). Effect of group electronegativity on spectroscopic, biological, chromogenic sensing and optical properties of 2-formyl-benzene sulfonic acid sodium salt-based Schiff bases. Journal of Molecular Structure, 1286, 135611. https://doi.org/10.1016/j.molstruc.2023.135611
You may like
Lastest Price from 2-Formylbenzenesulfonic acid sodium salt manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 1008-72-6
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 1000kg

US $1.00/KG2025-04-21
- CAS:
- 1008-72-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt