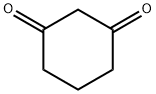
1,3-Cyclohexanedione: A Versatile Catalyst with Health and Environmental Considerations
General Description
1,3-Cyclohexanedione is a versatile cyclic diketone utilized as both a reactant and a catalyst in chemical transformations. Its structure allows for enhanced reactivity, particularly in non-aqueous environments, influencing reaction rates and outcomes based on concentration. While it has significant applications in catalysis, 1,3-cyclohexanedione poses health hazards, including acute toxicity via ingestion and serious eye damage. Additionally, 1,3-Cyclohexanedione is harmful to aquatic life, raising environmental concerns regarding its disposal and management. Understanding both its catalytic potential and associated risks is essential for optimizing its use and ensuring safety in industrial and laboratory settings.

Figure 1. 1,3-Cyclohexanedione
Multifaceted Role in Catalysis
Introduction to 1,3-Cyclohexanedione as a Catalyst
1,3-Cyclohexanedione is a cyclic diketone that plays a significant role in chemical synthesis and catalysis. Its unique structure allows it to be utilized not only as a reactant but also as a catalyst in various chemical transformations. The development of reactions involving 1,3-cyclohexanedione has revealed its versatility, particularly in non-aqueous environments. This diketone's α-methylene group enhances its reactivity, enabling it to participate in a variety of reactions, including nucleophilic additions and condensation reactions. As researchers investigate the catalytic properties of 1,3-cyclohexanedione, they discover that it can significantly influence reaction rates and outcomes by optimizing catalyst loading. 1
Effects of Concentration on Catalytic Activity
The concentration of 1,3-cyclohexanedione is crucial in determining the efficiency and effectiveness of catalytic reactions. Studies have shown that varying the amount of 1,3-cyclohexanedione can lead to significantly different reaction rates. For instance, in certain instances, higher concentrations of 1,3-cyclohexanedione resulted in slower reaction rates compared to lower concentrations. This phenomenon suggests that the buffering capacity of 1,3-cyclohexanedione can modulate the reaction environment, affecting both the loading of acid and base catalysts. The insightful adjustments in the concentration of 1,3-cyclohexanedione have enabled researchers to minimize the required catalyst loading while maintaining high yields and selectivities of the desired products. 1
Implications for Catalytic Reaction Development
The strategic application of 1,3-cyclohexanedione as a catalyst has significant implications for the development of new chemical reactions. By limiting the loading of 1,3-cyclohexanedione, researchers have successfully reduced the acidity of the catalyst to as low as 0.1 mol %. This reduction not only ensures that the reaction remains effective but also expands the reaction's scope, allowing for a broader range of products to be synthesized. The ability to fine-tune the amount of 1,3-cyclohexanedione used in reactions highlights its role as a multifunctional compound in catalysis. Therefore, understanding the behavior and influence of 1,3-cyclohexanedione in catalyzed reactions is vital for optimizing reaction conditions and improving overall efficiency in synthetic chemistry. 1
Toxicity
Health Hazards
1,3-Cyclohexanedione poses several health hazards that require attention in both industrial and laboratory settings. The compound is classified as harmful if swallowed, with acute toxicity levels suggesting an Oral LD50 for rats between 1,600 and 2,500 milligrams per kilogram. Ingestion of 1,3-cyclohexanedione can lead to serious health issues, necessitating strict handling protocols to prevent accidental consumption. Furthermore, direct contact with the substance can result in serious eye damage, underscoring the need for appropriate personal protective equipment when working with 1,3-cyclohexanedione. Its neurotoxic properties may also lead to acute solvent syndrome, which manifests through various neurological symptoms, thus further highlighting the need for caution. 2
Environmental Risks
In addition to its health hazards, 1,3-cyclohexanedione poses risks to the environment, particularly aquatic life. The compound has been identified as harmful to aquatic organisms, with long-lasting effects in the environment. This characteristic raises concerns about the proper disposal and management of 1,3-cyclohexanedione in industrial operations. Regulatory evaluations, such as those referenced by the Australian Inventory of Industrial Chemicals and the New Zealand Environmental Protection Authority, indicate that while 1,3-cyclohexanedione may not require extensive regulations for human health risks, its potential environmental impact cannot be overlooked. It is crucial for users of 1,3-cyclohexanedione to implement effective risk management strategies to mitigate both health and environmental hazards. 2
References:
[1] MUHAMMAD SOHAIL. Limiting the Loading of Reactant 1,3-Cyclohexanedione Enables the Use of Less Catalyst[J]. Journal of Organic Chemistry, 2023, 88 14: 9599-10324. DOI:10.1021/acs.joc.3c00838.See also
Lastest Price from 1,3-Cyclohexanedione manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 504-02-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00-0.00/KG2025-10-17
- CAS:
- 504-02-9
- Min. Order:
- 5KG
- Purity:
- 98%
- Supply Ability:
- 5000kg