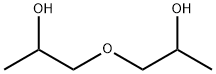
1,1'-Oxydi-2-propanol: main application, toxicology and carcinogenesis study
Introduction
1,1'-Oxydi-2-propanol (Figure 1) is a component of many widely used commercial products such as antifreeze, air fresheners, cosmetic products, solvents, and plastics. Toxicity and carcinogenicity studies of 1,1'-oxydi-2-propanol were performed because of suspected widespread consumer and occupational exposure based on its high production volume. The total production capacity of 1,1'-oxydi-2-propanol in the United States by its five major producers was 131 million pounds in 1998 [1]. The United States demand for 1,1'-oxydi-2-propanol in 1998 was 108 million pounds and was expected to increase to 125 million pounds by 2002, representing annual growth of 3–4%.

Main application
The major uses of 1,1'-oxydi-2-propanol are in the manufacture of plasticizers (38% of production), polyester resins (23% of production), cosmetics and fragrances (10% of production), polyurethane polyols (8% of production), and alkyd resins (7% of production) [1]. In cosmetic preparations, 1,1'-oxydi-2-propanol is used in hair care and bath products, perfumes, facial makeup, deodorants, and skin care products at concentrations ranging from less than 0.1–50%. Reported concentrations of 1,1'-oxydi-2-propanol in cosmetic formulations range from 0.1 to 1.5% in soaps, 0.01 to 0.15% in detergents, 0.05 to 0.5% in creams and lotions, and 1.0 to 8.0% in perfumes.[2]
1,1'-Oxydi-2-propanol is not used in drugs, pharmaceuticals, or food because its toxicologic characteristics have not been clearly defined [3]. It is an indirect food additive approved by the Food and Drug Administration for use as a component of adhesives used in packaging, transporting, or holding food and in cross-linked polyester resins used as articles or components of articles intended for repeated use in contact with food. The most probable routes of human exposure to 1,1'-oxydi-2-propanol are inhalation, dermal contact, and oral ingestion. Occupational exposure may occur through inhalation or dermal contact at sites where 1,1'-oxydi-2-propanol is produced or used. Consumers are exposed through the use of household products that contain 1,1'-oxydi-2-propanol such as air and room fresheners, household cleaners, cosmetic formulations, auto paints, and antifreeze. In addition, humans may be exposed to low levels of 1,1'-oxydi-2-propanol in contaminated groundwater and drinking water supplies. 1,1'-Oxydi-2-propanol was detected at concentrations of 0.2–0.4 ng/L in 2 of 11 drinking water samples taken from seven United States cities [2,4].
Exposure to 1,1'-Oxydi-2-propanol
The most probable routes of human exposure to 1,1'-oxydi-2-propanol are inhalation, dermal contact, and oral ingestion. Occupational exposure may occur through inhalation or dermal contact at sites where 1,1'-oxydi-2-propanol is produced or used. Consumers are exposed through the use of household products that contain 1,1'-oxydi-2-propanol such as air and room fresheners, household cleaners, cosmetic formulations, auto paints, and antifreeze. In addition, humans may be exposed to low levels of 1,1'-oxydi-2-propanol in contaminated groundwater and drinking water supplies. 1,1'-Oxydi-2-propanol was detected at concentrations of 0.2–0.4 ng/L in 2 of 11 drinking water samples taken from seven United States cities [4].
Toxicology and carcinogenesis study
Male and female F344/N rats and B6C3F1 mice were exposed to1,1'-oxydi-2-propanol in the drinking water for 2 weeks, 3 months, or 2 years. In the 2-week and 3-month studies, rats and mice were exposed to 0, 5000, 10,000, 20,000, 40,000, or 80,000 ppm 1,1'-oxydi-2-propanol. There was no mortality in the 2-week studies. In the 3-month rat study, all animals survived to the end of the study. Liver weights of rats exposed to 10,000 ppm or greater and kidney weights of rats exposed to 40,000 and 80,000 ppm were greater than those of the controls. The incidences of liver and kidney lesions were significantly increased in males exposed to 20,000 ppm or greater and females exposed to 80,000 ppm. Focal olfactory epithelial degeneration was present in all rats exposed to 80,000 ppm. In males, the incidences of testicular atrophy, epididymal hypospermia, and preputial gland atrophy were significantly increased in the 80,000 ppm group. In the 3-month mouse study, three males and one female exposed to 80,000 ppm died. Liver weights were increased, as was the incidence of centrilobular hypertrophy in males exposed to 40,000 ppm and males and females exposed to 80,000 ppm. In the 2-year studies, exposure groups were 0, 2500 (rats only), 10,000, 20,000 (mice only) or 40,000 ppm 1,1'-oxydi-2-propanol. Survival of male rats exposed to 40,000 ppm and mean body weights of males and females exposed to 40,000 ppm were significantly less than controls. In male rats, exposure to 1,1'-oxydi-2-propanol resulted in increased incidences and severities of nephropathy and secondary lesions in the parathyroid and forestomach. Increased incidences of focal histiocytic and focal granulomatous inflammation of the liver were also observed. In male and female rats, there were increased incidences of bile duct hyperplasia and changes in the olfactory epithelium of the nose. In mice, survival of males and females was similar to controls. Mean body weights and water consumption of males exposed to 40,000 ppm were less than that of the controls. Treatment-related nonneoplastic lesions did not occur in mice. Treatment-related neoplastic lesions did not occur in rats or mice.[2]
References
[1] ChemExpo, 2001. ChemExpo Profile Archives: Dipropylene Glycol. Maintained by the TurnAround Team, Inc. Retrieved 12 November 2001 from the World Wide Web: http://chemexpo.com/news/PROFILE980727.cfm.
[2] Hooth, M. J. , Herbert, R. A. , Haseman, J. K. , Orzech, D. P. , &Bucher, J. R. . (2004). Toxicology and carcinogenesis studies of dipropylene glycol in rats and mice. Toxicology, 204(2-3), 123-140.
[3] Patty’s Toxicology, 2001. In: Bingham, E., Cohrssen, B., Powell, C.H., (Eds.), EBST Glycols, fifth ed., vol. 7. Wiley, New York, pp. 35–36.
[4] Lin, D.C.K., Melton, R.G., Kopfler, F.C., Lucas, S.V., 1981. Glass capillary gas chromatographic/massspectrometric analysis of organic concentrates from drinking and advanced waste treatment waters. In: Keith, L.H. (Ed.), Advances in the Identification and Analysis of Organic Pollutants in Water, vol. 2. Ann Arbor Science Publishers, Ann Arbor, MI (pp. 861–906).
You may like
See also
Lastest Price from 1,1'-Oxydi-2-propanol manufacturers
US $131.00/kg2025-11-23
- CAS:
- 110-98-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 200kg
US $2.00-5.00/kg2025-06-13
- CAS:
- 110-98-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg