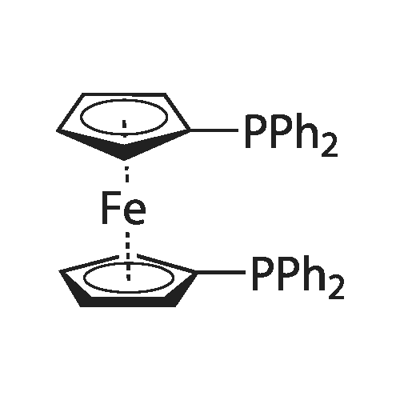
1,1'-Bis(diphenylphosphino)ferrocene: Properties and Coupling Reactions in Materials Syntheses
Ferrocenyl diphosphines and 1,1'-Bis(diphenylphosphino)ferrocene

Ferrocenyl diphosphines are among the most effective ligands for an astonishingly wide variety of transition metal-catalyzed transformations. 1,1'-Bis(diphenylphosphino)ferrocene (dppf ) is most commonly Ferrocenyl diphosphines material. Based on the impetus provided by the applications of the diphosphine 1,1 -bis(diphenylphosphino)ferrocene, the investigations directed towards the synthesis of new ferrocenylphosphines – sterically and/or electronically modified either at the ferrocenyl backbone or at the phosphorus atoms are still of fundamental and industrial interest.
Advantage
1,1'-Bis(diphenylphosphino)ferrocene is a stable, redox-active, conformationally flexible diphosphine in materials science, both as a reagent and a component. There are several reasons why dppf is so popular:
(i) its ease of synthesis from inexpensive ferrocene;
(ii) air, moisture and thermal stability;
(iii) ease of handling;
(iv) solubility in common solvents;
(v) conformational flexibility;
(vi) the stability and comparative ease of isolation of resulting Group 6–12 metal complexes;
(vii) easy oxidation of the ferrocene moiety;
(viii) ESI-MS, NMR and XRD-friendly functionality and, most importantly,
(ix) the comparatively high success rate among the common phosphines and diphosphines in catalysis.
Coupling reactions profile
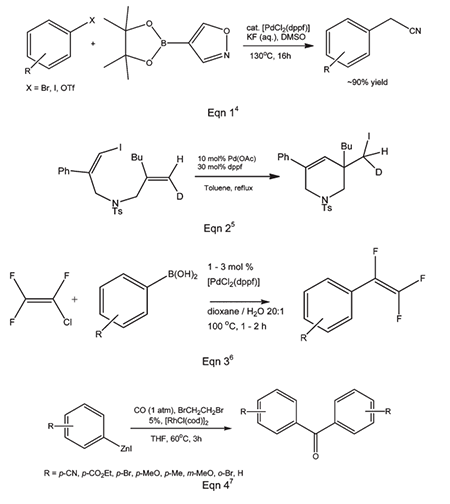
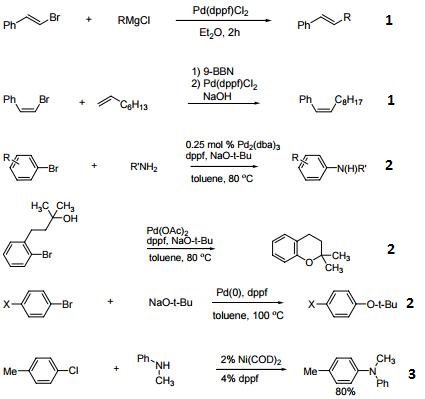
The ferrocenyl backbone gives a large natural bite angle to dppf as a diphosphine. The computationally calculated preferred P–M–P bite angle for dppf is 95.60° compared to an average P–M–P angle of 98.74° determined from X-ray crystal structures.3 However, if a bidentate ligand is to stabilize intermediates of different geometry and electron density [e.g. from Pd(0) to Pd(II)] during oxidative addition and reductive elimination, then a low energy barrier to the large expansion or contraction of the P–M–P angle is an advantage. The ferrocene backbone offers the flexibility to accommodate open trigonal planar geometries and more compressed square planar, trigonal bipyramidal and octahedral intermediate complexes with a minimal energy penalty. This versatility results in both the stabilization of a wide range of catalytically active transition states and also a plethora of metal– dppf materials with nuclearity ranging from unity to essentially infinity. Dppf figures prominently in catalytic C–B, C–C, C–N, C–O, C–P and C–halide coupling reactions with metals including Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Au and Zn. Modern catalysis studies of this type usually report a comparison of results with different mono- and bidentate phosphine ligands, and dppf is almost invariably included.&c;
Indeed, the importance of 1,1'-Bis(diphenylphosphino)ferrocene in modern carbon–carbon coupling catalysis might challenge the place traditionally attributed to the triphenylphosphine ligand PPh3 in the future. A reason for this success is probably its easy access and commercial availability. Nowadays, many dppf derivatives have been produced and are readily available.
References:
[1] AZIZ FIHRI J C H Philippe Meunier. Performances of symmetrical achiral ferrocenylphosphine ligands in palladium-catalyzed cross-coupling reactions: A review of syntheses, catalytic applications and structural properties[J]. Coordination Chemistry Reviews, 2007, 251 15: 1951-2102. DOI:10.1016/j.ccr.2007.03.020.[2] YOUNG D J, CHIEN S W, HOR T S A. 1,1’-Bis(diphenylphosphino)ferrocene in functional molecular materials[J]. Dalton Transactions, 2012, 41: Page 12637 to 12992. DOI:10.1039/C2DT31271A.
You may like
Related articles And Qustion
Lastest Price from 1,1'-Bis(diphenylphosphino)ferrocene manufacturers
US $0.00/kg2025-10-21
- CAS:
- 12150-46-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 2000kgs

US $390.00-238.00/KG2025-04-21
- CAS:
- 12150-46-8
- Min. Order:
- 0.1KG
- Purity:
- 99% HPLC
- Supply Ability:
- 100KG