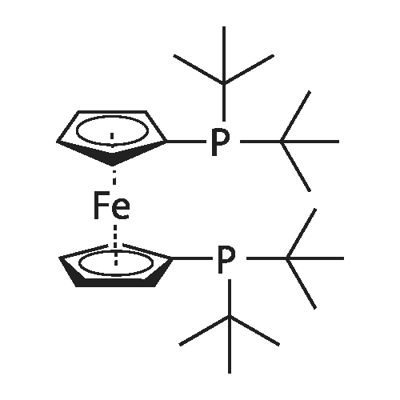
1,1'-Bis(di-tert-butylphosphino)ferrocene: Anodic Electrochemistry, Application in Catalysis and its Preparation Method
General Description
1,1'-Bis(di-tert-butylphosphino)ferrocene is a notable ligand in anodic electrochemistry and catalysis, exhibiting unique electrochemical behavior due to its bulky substituents and interactions with transition metals. Cyclic voltammetry studies reveal complex oxidation processes for its metal complexes, particularly when paired with chalcogenides. In catalysis, 1,1'-Bis(di-tert-butylphosphino)ferrocene enhances palladium-catalyzed reactions, improving yields in Suzuki coupling without external bases. Its preparation involves reacting ferrocene with diaryl or dialkyl phosphine oxides under controlled conditions, followed by hydrolysis and crystallization. The gentle reaction conditions make it suitable for industrial applications in organic synthesis and advanced electrochemical systems.

Figure 1. 1,1'-Bis(di-tert-butylphosphino)ferrocene
Anodic Electrochemistry
Introduction to Anodic Electrochemistry
The anodic electrochemistry of 1,1'-Bis(di-tert-butylphosphino)ferrocene involves the exploration of its oxidative behavior in methylene chloride, utilizing tetrabutylammonium hexafluorophosphate as a supporting electrolyte. This ferrocene derivative, known for its bulky di-tert-butylphosphino substituents, offers a distinct electrochemical profile characterized by its robustness and stability under electrochemical conditions. The study extends to its interactions when bound to transition metals, forming complexes that exhibit unique electrochemical properties compared to the free 1,1'-Bis(di-tert-butylphosphino)ferrocene molecule. These properties are primarily investigated through techniques such as cyclic voltammetry, which provides detailed insights into the electrochemical behaviors and the potential applications of these complexes in various electrochemical systems. 1
Electrochemical Analysis and Complex Formation
In the context of 1,1'-Bis(di-tert-butylphosphino)ferrocene, the formation of complexes with transition metals leads to notable differences in electrochemical characteristics. Cyclic voltammetry studies reveal that these complexes, along with chalcogenides like 1,1'-Bis(di-tert-butylphosphino)ferroceneS2 and 1,1'-Bis(di-tert-butylphosphino)ferroceneSe2, exhibit diverse oxidation mechanisms. For instance, 1,1'-Bis(di-tert-butylphosphino)ferroceneS2 undergoes a simple one-electron oxidation process centered around the iron moiety. Conversely, the oxidation of 1,1'-Bis(di-tert-butylphosphino)ferroceneSe2 is not only irreversible but also complex, proceeding through an EE (electrochemical-electrochemical) mechanism, highlighting a more intricate interaction involving selenium centers. These findings underscore the significant impact of the chalcogen elements on the electrochemical properties of the 1,1'-Bis(di-tert-butylphosphino)ferrocene complexes, potentially paving the way for their application in advanced electrochemical devices and systems. 1
Application in Catalysis
1,1'-Bis(di-tert-butylphosphino)ferrocene has emerged as a valuable ligand in catalysis, particularly in transition metal-catalyzed reactions. In the context of the Suzuki coupling reaction, this diphosphine ligand enhances the catalytic activity of palladium catalysts. The robust and bench-stable characteristics of 1,1'-Bis(di-tert-butylphosphino)ferrocene make it particularly effective for cross-coupling processes. The ligand provides excellent ligand field stabilization to palladium, leading to improved yields and selectivity in reactions involving aryl and heteroaryl boronic acids. Its steric and electronic properties facilitate the activation of aryl halides, significantly enhancing the efficiency of the catalytic cycle. 2
Mechanism and Efficiency
The catalytic system utilizing 1,1'-Bis(di-tert-butylphosphino)ferrocene demonstrates remarkable versatility in various reactions, including both tandem and classical Suzuki coupling. When combined with palladium(II) catalysts, the ligand promotes efficient aryl-aryl bond formation through a variety of nucleophilic precursors, such as Grignard reagents and boronates. Additionally, the conditions of the reaction can often be carried out without the need for external bases, streamlining the process and improving overall yields. Due to its effectiveness, 1,1'-Bis(di-tert-butylphosphino)ferrocene is increasingly employed in the development of pharmaceutical compounds and organic materials, solidifying its role as an essential catalyst in modern organic synthesis. 2
Preparation Method
The preparation method of 1,1'-Bis(di-tert-butylphosphino)ferrocene involves several key steps, executed under controlled conditions to ensure the high yield and purity of the product. Initially, ferrocene is mixed with a solvent, specifically 1,2-dichloroethane, and either diarylphosphine oxide or dialkylphosphine oxide in a dry reactor. This mixture is conducted under the protection of an inert gas to prevent unwanted reactions. The catalyst, boron trifluoride diethyl etherate, is then gradually added at temperatures ranging from -10 to 10°C. Following the addition of the catalyst, the reaction mixture is heated to a temperature between 60-80°C to facilitate the reaction. After the completion of the reaction, the mixture is cooled and water is added to undergo hydrolysis, which helps in breaking down any excess reagents. The product is then extracted, dried, and crystallized to obtain 1,1'-Bis(di-tert-butylphosphino)ferrocene tetrafluoroborate. To achieve the final 1,1'-Bis(di-tert-butylphosphino)ferrocene product, a deprotection step is carried out by heating the intermediate in methanol. This method is noted for its mild reaction conditions and is deemed suitable for industrial production, making 1,1'-Bis(di-tert-butylphosphino)ferrocene a viable ligand for metal catalysts in various applications, including organic photoelectric materials and medicinal chemistry. 3
References:
[1] FAWN N. BLANCO. Anodic Electrochemistry of Free and Coordinated 1,1‘-Bis(di-tert-butylphosphino)ferrocene[J]. Organometallics, 2006, 25 18: 4235-4432. DOI:10.1021/om051011+.[2] MAREK ?UBI?áK T T Václav Eigner. Bench‐Stable Sulfoxide‐Based Boronates: Preparation and Application in a Tandem Suzuki Reaction[J]. Advanced Synthesis & Catalysis, 2018, 360 23: 4459-4649. DOI:10.1002/adsc.201801000.
Related articles And Qustion
Lastest Price from 1,1'-Bis(di-tert-butylphosphino)ferrocene manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 84680-95-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00/g2025-04-12
- CAS:
- 84680-95-5
- Min. Order:
- 500g
- Purity:
- 98%
- Supply Ability:
- 10