(R)-3-Amino-1-Butanol: Applications and Biosynthesis Method
General Description
(R)-3-Amino-1-Butanol is a key building block in pharmaceutical synthesis, particularly in the production of Dolutegravir for HIV treatment, showcasing its therapeutic significance. Beyond Dolutegravir, (R)-3-Amino-1-Butanol is also used in penicillium antibiotics and the anti-tumor drug 4-methylcyclophosphamide. Traditional chemical synthesis faced challenges like low yields and environmental concerns, prompting the exploration of ruthenium-catalyzed methods, which also had limitations. A breakthrough came with the use of a novel transaminase from Actinobacteria sp. that enabled efficient biosynthesis, achieving a high yield and enantiomeric excess, marking a significant advancement in sustainable production methods for (R)-3-Amino-1-Butanol.

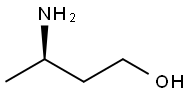
Figure 1. (R)-3-Amino-1-Butanol
Applications
Pharmaceutical Synthesis and Therapeutic Applications
(R)-3-Amino-1-Butanol is a crucial building block in the synthesis of various pharmaceuticals. It plays a significant role in the production of Dolutegravir, a cutting-edge integrase inhibitor used in treating HIV infection. Dolutegravir is renowned for its effectiveness in managing HIV/AIDS due to its ability to combine the therapeutic effects of multiple anti-HIV/AIDS drugs. This integration allows Dolutegravir to operate independently of additional drug enhancers, distinguishing it from traditional anti-HIV/AIDS medications. The use of Dolutegravir also demonstrates excellent efficacy with minimal risk of treatment-limiting toxicities, making (R)-3-Amino-1-Butanol an essential compound in this context. 1
Broader Pharmaceutical Uses
Beyond Dolutegravir, (R)-3-Amino-1-Butanol is also utilized in the synthesis of penicillium antibiotics and the anti-tumor drug 4-methylcyclophosphamide. These applications underscore the compound's versatility and importance in drug development. Penicillium antibiotics are critical for treating bacterial infections, while 4-methylcyclophosphamide serves as an effective anti-tumor agent. The significant role of (R)-3-Amino-1-Butanol in these diverse pharmaceutical applications highlights its value in both therapeutic and preventive medicine, illustrating its broad impact on medical science and drug formulation. 2
Biosynthesis Method
Traditional Chemical Synthesis
Initially, the production of (R)-3-amino-1-butanol was primarily achieved through a chemical synthesis method involving the reaction of (R)-(+)-1-phenylethylamine with crotonates. This process generated a mixture of epimers due to the presence of two chiral centers. The subsequent separation of these epimers was carried out using silica gel column chromatography. The isolated single isomer underwent further reduction and debenzylation to yield (R)-3-amino-1-butanol. However, this chemical method faced several issues including low yield and product purity, high consumption of organic solvents, and the use of expensive reducing agents like lithium aluminum hydride. These factors resulted in poor economic feasibility and environmental concerns associated with the synthesis of (R)-3-amino-1-butanol.
Development of Ruthenium-Catalyzed Synthesis
To address the limitations of the traditional chemical synthesis, a novel approach involving a ruthenium complex was developed. This method catalyzed the hydrogen reduction of (R)-3-aminobutyric acid ester to produce (R)-3-amino-1-butanol. Although this route simplified the synthesis process with fewer catalytic steps, it encountered challenges such as the high cost and limited availability of the chiral substrate, (R)-3-aminobutyric acid ester. Additionally, the method exhibited low enantiomeric excess and yield of the final product, which restricted its industrial application. Despite these advancements, the search for more efficient and sustainable methods continued, leading to the exploration of biocatalytic approaches for the synthesis of (R)-3-amino-1-butanol.
Biocatalytic Synthesis Using Transaminases
Biocatalysis emerged as a promising alternative to traditional chemical methods for synthesizing (R)-3-amino-1-butanol. The use of transaminases, which are enzymes capable of catalyzing the transfer of amino groups, showed significant potential. However, many transaminases exhibit (S)-specificity, making the production of (R)-amines challenging. A breakthrough came with the discovery and application of a novel transaminase from Actinobacteria sp. (As-TA), which successfully catalyzed the biosynthesis of (R)-3-amino-1-butanol. By transferring the amino group from isopropylamine to 4-hydroxy-2-butanone under optimized conditions, the process achieved a remarkable conversion rate and enantiomeric excess, with a maximum yield of 29.6 g/L and 99.9% enantiomeric excess. This development marked the first successful biosynthesis of (R)-3-amino-1-butanol using a transaminase, significantly enhancing the enzyme pool with (R)-specific transaminases and demonstrating a more sustainable approach to producing this valuable intermediate. 2
References:
[1] XIAO-LING TANG . Efficient biosynthesis of (R)-3-amino-1-butanol by a novel (R)-selective transaminase from Actinobacteria sp.[J]. Journal of biotechnology, 2019, 295: 1-90. DOI:10.1016/j.jbiotec.2019.02.008.You may like
See also
Lastest Price from (R)-3-amino-1-butanol manufacturers
US $0.00-0.00/kg2025-12-02
- CAS:
- 61477-40-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000
US $0.00-0.00/KG2025-12-02
- CAS:
- 61477-40-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS